Add your feed to SetSticker.com! Promote your sites and attract more customers. It costs only 100 EUROS per YEAR.
Pleasant surprises on every page! Discover new articles, displayed randomly throughout the site. Interesting content, always a click away
ENDECE
New Drug ConceptsNDC-1308: A Novel Therapeutic For Acute Respiratory Distress Syndrome (ARDS) Including COVID-19 Patients 21 Jan 2021, 7:29 pm
As SARS-Cov-2 / COVID-19 continues to dominate our lives, the world is looking for a way to get back to “Normal”. Unfortunately, “Normal”, is a long ways off and may have to be redefined in general. Public health officials are confident that broad vaccination will move us in the right direction. This is reinforced by recent comments from the new Biden Administration. However, better – safer and more effective – medicines (therapeutics) like NDC-1308 are a necessity. Most COVID-19 patients die from Acute Respiratory Distress Syndrome (ARDS) and not from the SARS-CoV-2 infection itself. Providing better health outcomes and reducing mortality for this group of COVID-19 patients remains an unmet medical need and an important societal need.
As presented in a recent article in The Atlantic (“The Next Phase of Vaccination Will Be Even Harder,” Sarah Zhang, January 7, 2021), there are unresolved issues with vaccines for SARS-CoV-2 and its emerging variants:
NDC-1308 has the ability to directly reduce inflammation, hyperinflammation and the “cytokine storm” within patients’ lungs, and therefore, reduce the risk of developing acute respiratory failure. In addition to treating the direct effects of ARDS in patients, ENDECE data shows NDC-1308 is also capable of reducing the SARS-CoV-2 viral load within the lungs as it has the potential to reduce ACE2 receptors threefold. ACE2 receptors are the point-of-entry for the SARS-CoV-2 virus into cells.
ENDECE is excited about the promise of NDC-1308 for the treatment of Acute Respiratory Distress Syndrome (ARDS) and specifically as a much needed treatment of ARDS in COVID-19 patients.
The post NDC-1308: A Novel Therapeutic For Acute Respiratory Distress Syndrome (ARDS) Including COVID-19 Patients appeared first on ENDECE.
NDC-1308 A Therapeutic for COVID-19 And Other Pulmonary Viruses 12 Nov 2020, 9:51 pm
To win the battle with SARS-CoV-2 we need a therapeutic designed to regain control of immune response. Historically vaccines have played an important role in the prevention of many diseases. However, the overall effect of a vaccine within society depends on both efficacy and percent of the population that gets vaccinated.
For example, if the Pfizer vaccine is 90% effective, as projected, and half of the population receives the vaccine, over 50% of the population will remain unprotected.
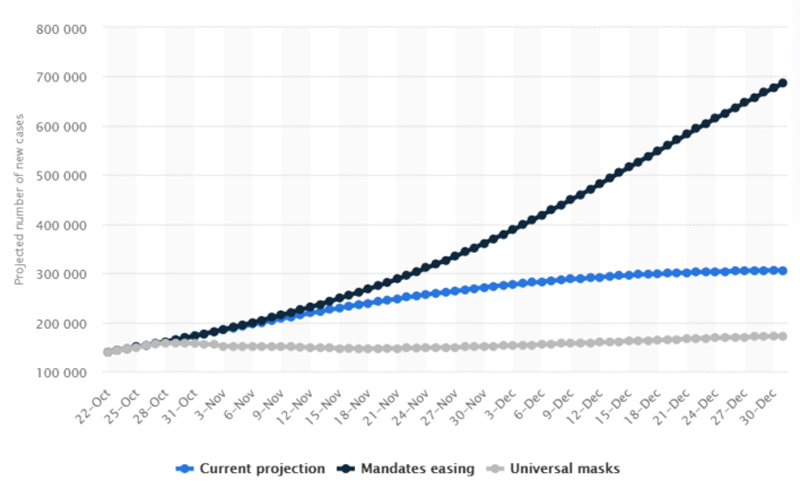
Statistically, by easing compliance with mandates like wearing a mask, it is projected that up to 700,000 new cases of COVID-19 may take place in the US by December 31 of this year, per day.
https://www.statista.com/statistics/1176657/covid-projected-new-cases-per-day-by-scenario-us/
SAVRS-CoV-2 is an aggressive virus that thrives in the pulmonary system and leads to COVID-19. It overwhelms the body’s natural immune system, causes respiratory distress, respiratory failure, and potentially death. In addition to a vaccine, therapeutics are needed to reduce inflammation and viral load within the pulmonary system.
ENDECE did just that. We developed NDC-1308, a therapeutic that works at the gene level to reduce inflammation and viral load within the pulmonary system. Either alone or in combination with a vaccine, NDC-1308 may help to win the battle against viruses like SARS-CoV-2 / COVID-19 as well as any future SARS and pulmonary viruses causing Acute Respiratory Distress Syndrome (ARDS).
The post NDC-1308 A Therapeutic for COVID-19 And Other Pulmonary Viruses appeared first on ENDECE.
NDC-1308 A NOVEL THERAPEUTIC FOR COVID-19 21 Oct 2020, 8:54 pm
We need both vaccines and therapeutics as not everyone will be immunized effectively against SARS-CoV-2 / COVID-19
The search for a SARS-CoV-2 vaccine continues to be of high priority. However, there are several aspects of SARS-CoV-2 and other viruses, such as the seasonal flu, which a vaccine cannot fully address. These include long lasting effects to the lungs, heart, and other organs caused by inflammation and high viral load, even in individuals who are asymptomatic.
Let’s look at the seasonal flu for a moment. The flu vaccine is a preventative measure (1) that has saved many lives, and should be received every year. With that said, for the 2019-2020 flu season, only 53% of Americans (2) were vaccinated. On top of that, the vaccine was only 44% effective (3), meaning approximately 23% of the American population was protected. Finally, the vaccine is only effective for 6-9 months.
Because vaccines are not 100% effective, an outbreak like COVID-19, which has already killed over 220,000 US citizens within 9 months, requires other treatment options. Attacking the virus from multiple directions is critically important.
ENDECE developed NDC-1308, a novel therapeutic for COVID-19 designed to reduce inflammation in the lungs as well as the number of ACE2 receptors in the pulmonary system, reducing the viral load. By reducing pulmonary inflammation and viral load, as well as preventing cytokine storm due to viral infections like SARS-CoV-2 / COVID-19, NDC-1308 should reduce long term effects, keep people off ventilators, and even prevent death.
The post NDC-1308 A NOVEL THERAPEUTIC FOR COVID-19 appeared first on ENDECE.
NDC-1308 (MC1) Therapeutic for Seasonal Viral Influenza 9 Jul 2020, 8:08 pm
Within the US alone, there are over 200,000 hospitalizations annually from seasonal viral influenza. Patients requiring mechanical ventilation have a high mortality rate of 20% or over 40,000 people annually.
The World Health Organization (WHO) estimates that influenza impacts between 3 Million and 5 Million people worldwide causing between 250,000 and 500,000 deaths annually from respiratory distress and failure. These deaths do not include other respiratory diseases such as adenovirus, rhinovirus, hMPV, PIV, RSV etc.
NDC-1308 (MC1) has a known MOA that directly shifts pro-inflammatory M1 macrophages to the anti-inflammatory M2 state, and is agnostic to the type of virus infecting and inflaming the lungs. Therefore, there is a strong need for NDC-1308 (MC1) to treat lung inflammation beyond SARS-CoV-2, COVID-19, and future Coronaviruses. NDC-1308 (MC1) will be an important treatment for all viral respiratory diseases, preventing progression to respiratory distress, respiratory failure, and death.
The post NDC-1308 (MC1) Therapeutic for Seasonal Viral Influenza appeared first on ENDECE.
Differences Between NDC-1308 (MC1) and Dexamethasone 8 Jul 2020, 10:36 pm
The differences between NDC-1308 (MC1) and Dexamethasone proposed Mechanism of Action (MOA) support NDC-1308 (MC1) may have increased efficacy compared to Dexamethasone in all COVID-19 patient groups. By addressing the early signs of lung inflammation in COVID-19 patients, NDC-1308 (MC1) is projected to reduce the amount of time people spend in hospitals and prevent the use of a ventilator. Compared to Dexamethasone, NDC-1308 (MC1) shows substantial improvement to survival in COVID-19 patients.
NDC-1308 (MC1) has a single Mechanism of Action (MOA) for reducing lung inflammation that can be efficacious in all COVID-19 patient groups, as well as many other inflammatory lung viruses.
Proposed MOA
Dexamethasone
NDC-1308 (MC1)
T Cell Depletion

Dexamethasone
T cell depletion without macrophage polarization (Biomaterials 2015, Jan; 37: 367-382)

NDC-1308 (MC1)
None
Reduction in M1 Macrophages

Dexamethasone
Anti-inflammatory macrophages, but MOA is unknown
Reduction in numbers of M1 macrophages caused by dexamethasone binding to mineralocorticoid receptor (MR) in lung alveolar cells; may competitively inhibit aldosterone binding to the MR; aldosterone binding to MR causes upregulation of hyper-inflammatory M1 macrophages due to Ace1/Ace2 dysregulation from SARS-CoV-2 binding to Ace2 receptors (would result in increased numbers of pro-inflammatory Ace1 receptors and M1 macrophages)
(Critical Care, 2020, 24:318)

NDC-1308 (MC1)
Macrophage polarization to M2, anti-inflammatory state by directly upregulating gene and protein expression for lipoprotein lipase (LPL) and ApoC2 within lung macrophages
Clinical Studies

Dexamethasone
Reduces mortality in COVID-19 patients on ventillators by 33%, and by 20% in patients receiving oxygen

NDC-1308 (MC1)
Progressing to Phase 1 Clinical studies in COVID-19 patients

Pre-IND meeting completed with FDA
The post Differences Between NDC-1308 (MC1) and Dexamethasone appeared first on ENDECE.
Macrophage Inflammation In The Lungs 3 Jul 2020, 7:19 pm
WHAT IS A MACROPHAGE?

A macrophage is a type of white blood cell called a phagocyte. It is the first line of defense that cleans the body of bacteria and viruses called antigens.
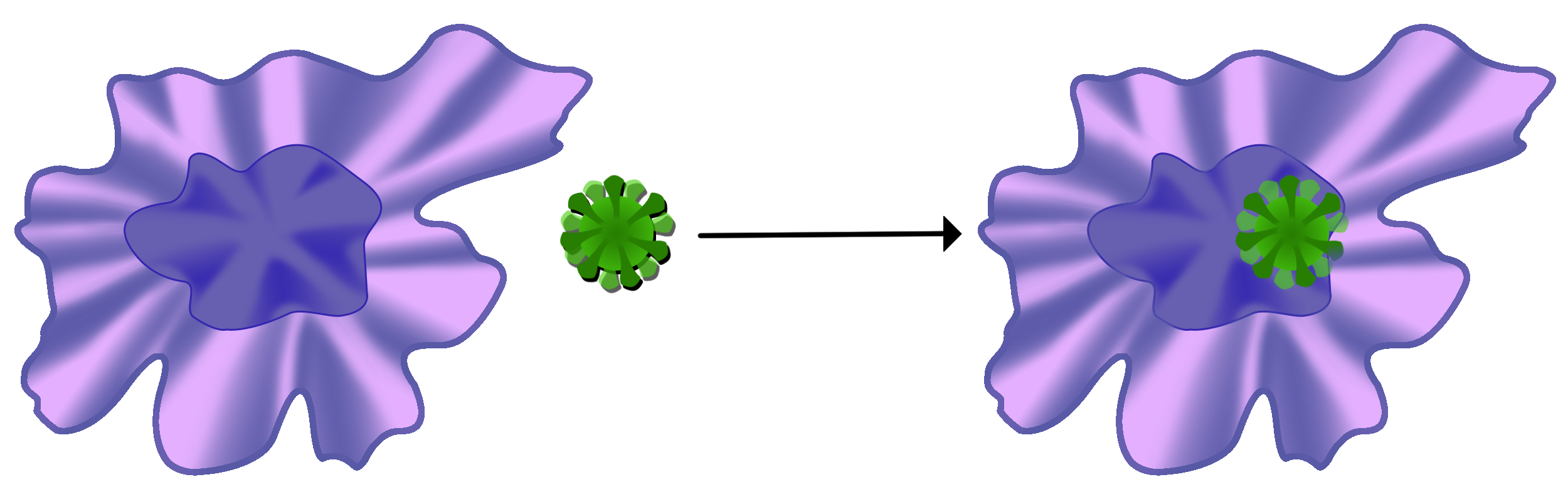
When a macrophage locates an antigen, it engulfs and destroys it.
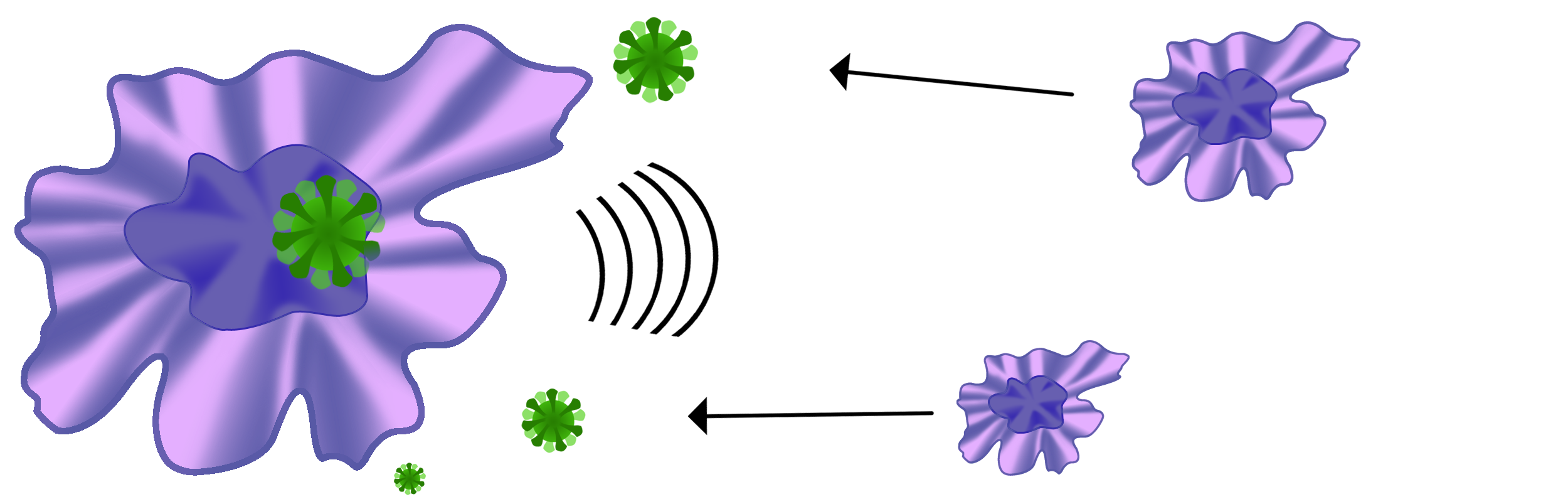
Upon being presented with numerous antigens, macrophages send out inflammatory signals that alert other macrophages to migrate to the area in order to help destroy the bacteria or virus.
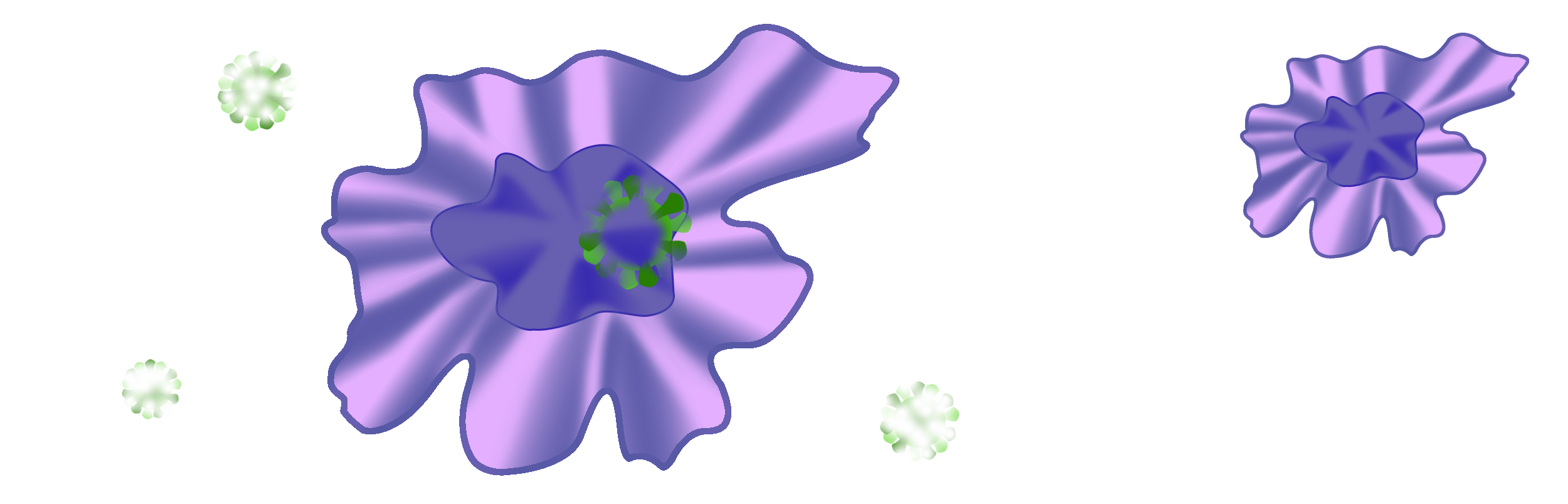
Normally when the antigens are destroyed, the macrophages stop producing inflammatory signals and return to a resting state.
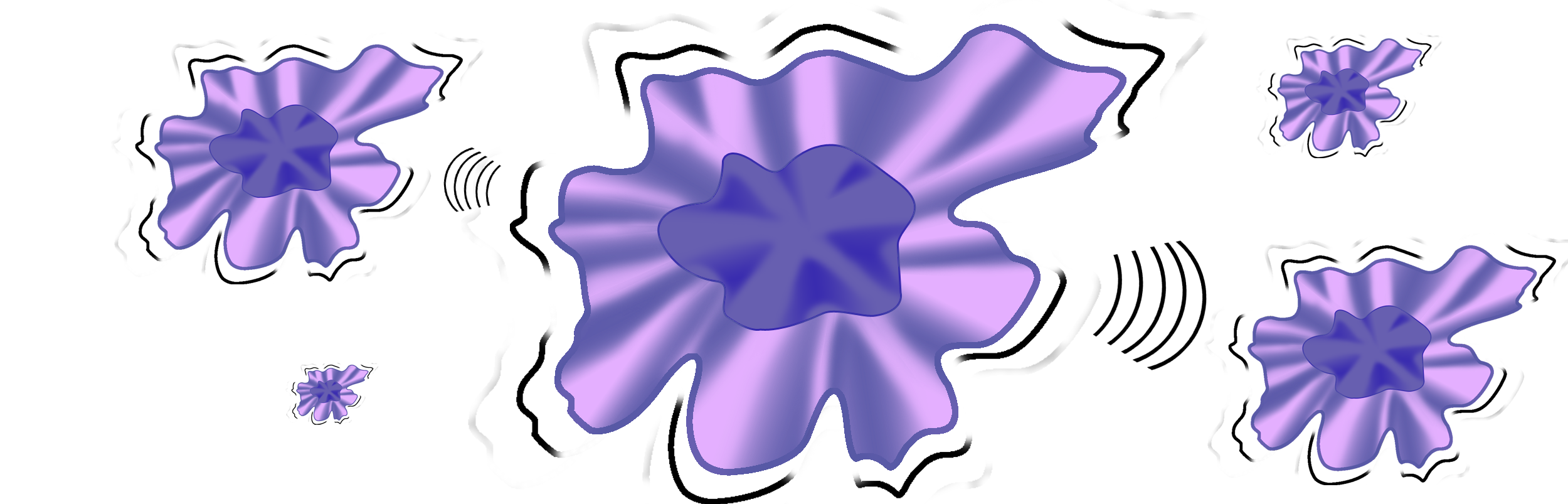
When macrophages do not return to their resting state after the antigens have been destroyed, and continue to produce inflammatory signals, the prolonged inflammation can lead progressively to respiratory distress, respiratory failure, Acute Respiratory Distress Syndrome (ARDS), and the Cytokine Storm, as well as death.
The post Macrophage Inflammation In The Lungs appeared first on ENDECE.
ENDECE PRESENTS AT MICHAEL, MARCIA, and CHRISTA PARSEGHIAN SCIENTIFIC CONFERENCE FOR NPC RESEARCH | VIRTUAL SYMPOSIUMS 10 Jun 2020, 10:13 pm
On Monday, June 1, ENDECE presented the following abstract to the Michael, Marcia, and Christa Parseghian Scientific Conference for NPC Research | Virtual Symposiums
NDC-1308: A novel anti-inflammatory and remyelinating therapeutic in late pre-clinical studies for stopping / slowing disease progression and reversing functional losses in patients diagnosed with Niemann-Pick Disease type C (NPC)
Dr. James Yarger and James Hoyes, ENDECE, LLC, Mequon, WI 53092
A primary cause of Niemann-Pick Disease type C (NPC) is loss-of-function mutations in either NPC1 or NPC2 genes leading to a disruption in cholesterol and lipid transport out of endosomes and lysosomes. The accumulation of free cholesterol within the endosomes/lysosomes leads to progressive levels of neurodegeneration. Two key pathologies that drive this neurodegenerative process in NPC are 1) chronic, progressive neuroinflammation and 2) demyelination/hypomyelination.
ENDECE has discovered and is developing NDC-1308 as a novel anti-inflammatory and remyelinating small molecule therapeutic to stop/slow disease progression and reverse functional losses in patients diagnosed with NPC. NDC-1308 activity is derived from its ability to upregulate key genes, and their respective proteins, in signaling pathways representing both pathologies within central nervous system (CNS) tissues. NDC-1308 has a dual mechanism of action. First, NDC-1308 directly polarizes macrophages to the M2-like, anti-inflammatory/prorepair state by upregulating the lipoprotein lipase (LPL) gene and functional protein expression within CNS macrophages. This ability to polarize macrophages to the M2-like state is important since the first evidence of neuroinflammation in the NPC mouse is early activation of microglia (M1-like, pro-inflammatory macrophages) which begins the neurodegenerative cascade (Baudry et al 2003; Smith et al 2009; Cologna et al 2014). Second, NDC-1308 directly induces oligodendrocyte progenitor cells (OPCs) to differentiate into mature myelinating oligodendrocytes by upregulating key genes and functional proteins (Olig2, MBP, MOG, DNER) within CNS OPCs which induce remyelination of demyelinated/hypomyelinated axons. This ability to induce remyelination is important since progressive Purkinje cell degeneration is an important neuropathological feature of NPC (Pentchev et al 1984; Higashi et al,1993) along with myelin loss in the pre-frontal cortex, corpus callosum and hippocampus in NPC 1 mice (Yan et al 2011; Caporali et al 2016).
ENDECE has completed several important pre-clinical studies that support this novel therapeutic profile. M2- polarization with NDC-1308 has been confirmed in neonatal primary rat microglia cultures. In addition, prophylactic dosing in EAE mouse models demonstrates NDC-1308 provides dose-dependent, anti-inflammatory benefits consistent with an M2-like macrophage phenotype. Induced remyelination has been confirmed using the cuprizone mouse model. In this model, NDC-1308 reproducibly demonstrates remyelination of the pons, hippocampus, and cortex consistent with OPC differentiation into mature oligodendrocytes.
Importantly, NDC-1308 demonstrates a strong safety profile; it does not upregulate the expression of 38 genes known to be involved in cell proliferation and migration (i.e. has a low carcinogenic profile). Nor does NDC-1308 appear to be uterotropic, mutagenic, genotoxic, or pro-arrhythmic. An SBE-β-cyclodextrin based formulation has been developed for nasal administration of NDC-1308 across the olfactory epithelium into the brain and spinal cord. We believe NDC-1308 possesses many desirable attributes of an effective NPC therapy and we are ready to progress NDC-1308 into IND-enabling and then Phase 1/2 clinical studies.
Baudry, M et al, 2003, Exp Neurol, 184:887-903
Caporali P et al, 2016, Acta Neuropathol Comm, 4:94
Cologna, SM et al, 2014, J Inherit Metab Dis, 37:83-92
Higashi Y et al, 1993, Acta Neuropathol 85:175-184
Pentchev PV et al, 1984, J Biol Chem 259:5784-5791
Smith, D et al, 2009, Neurobiol Dis, 36:242-251
Yan X et al, 2011, Metab Brain Dis, 26:29-306
ENDECE, LLC. Data on File.
The post ENDECE PRESENTS AT MICHAEL, MARCIA, and CHRISTA PARSEGHIAN SCIENTIFIC CONFERENCE FOR NPC RESEARCH | VIRTUAL SYMPOSIUMS appeared first on ENDECE.
ENDECE ANNOUNCES LEAD CLINICAL DEVELOPMENT CANDIDATE NDC-1308 (MC1) AS A THERAPEUTIC FOR SARS-CoV-2 AND COVID-19 3 May 2020, 7:36 pm
ENDECE’s lead clinical development candidate, NDC-1308 (MC1) could prove to be an important therapeutic option in minimizing respiratory failure in COVID-19 patients caused by the SARS-CoV-2 virus health emergency and global pandemic.
ENDECE’s Research Effort to Treat COVID-19 Patients
ENDECE is developing a new drug that addresses the deterioration in pulmonary function caused by chronic inflammation in lung tissue and the subsequent risk of acute respiratory failure. Chronic inflammation, a pathology commonly found in many disease states including COVID-19, can lead to respiratory failure and death. This condition is part of Acute Respiratory Distress Syndrome (ARDS).
Pre-clinical research shows that NDC-1308 (MC1), works by directly up-regulating the Lipoprotein Lipase (LPL) gene and functional protein expression, polarizing macrophages to the pro-repair, anti-inflammatory M2-like state. This specific part of the overall mechanism of action of NDC-1308 (MC1) reduces inflammation. ENDECE predicts that NDC-1308 (MC1) could be safely administered via inhalation therapy and could become an important therapeutic approach to minimizing the acute, severe inflammatory response within lung tissue. ENDECE’s approach could significantly reduce the incidence of, or even prevent, acute respiratory failure associated with ARDS and result in a reduction in the number of COVID-19 patients on ventilators and / or reduce “on-ventilator time” in the ICU setting.
A manufacturing process for NDC-1308 (MC1), a small molecule, is scalable and ready to produce quantities required to begin expedited clinical studies in patients suffering from COVID-19. ENDECE is actively pursuing government funding and equity investment to expedite clinical development during this ongoing pandemic.
About ENDECE
ENDECE is a biopharmaceutical company focused on the discovery and development of new therapeutics that stop disease progression, restore function, and improve the lives of people suffering from neurodegenerative and inflammatory diseases. ENDECE is fully committed to creating a healthier future and is proud to serve our community, country and the world.
The post ENDECE ANNOUNCES LEAD CLINICAL DEVELOPMENT CANDIDATE NDC-1308 (MC1) AS A THERAPEUTIC FOR SARS-CoV-2 AND COVID-19 appeared first on ENDECE.