Add your feed to SetSticker.com! Promote your sites and attract more customers. It costs only 100 EUROS per YEAR.
Pleasant surprises on every page! Discover new articles, displayed randomly throughout the site. Interesting content, always a click away
CJ Industries
CJ IndustriesUnlocking the Power of Ownership: C&J Industries’ ESOP Journey 21 Jan 2025, 7:33 pm
Back in 1962, Harold Corner and Dick Johnston launched their dream venture, Meadville Precision Tool & Mold, from a small garage. They quickly became well-renowned for building high-precision plastic injection molding components and quality injection molds. Their expansion into the medical and business machine markets fueled even further growth in the 1980s, prompting a new name–C&J Industries, Inc., in honor of the founders.
The Birth of the ESOP
The 1980s marked a turning point. When founder Harold Corner retired, Dick Johnston proposed an Employee Stock Ownership Plan (ESOP) to keep the company in trusted hands. Fast forward to 2016, as long-standing CEO, Dennis Frampton announced his retirement and disclosed that C&J’s Board of Directors had sold their shares and created an Employee Stock Ownership Plan—making C&J a 100% employee-owned company. The ESOP accomplished two important goals: ensuring that C&J would remain in Meadville, PA, and securing a promising future for C&J employees.
Why an ESOP? Empowerment in Ownership
 The ESOP at C&J is more than just shares – it’s about empowerment. Every employee has a voice in the company’s future. Major decisions, from business acquisitions to potential sales, require majority employee approval. In this model, C&J employees aren’t just team members; they have invested in every success, every customer interaction, and every innovation.
The ESOP at C&J is more than just shares – it’s about empowerment. Every employee has a voice in the company’s future. Major decisions, from business acquisitions to potential sales, require majority employee approval. In this model, C&J employees aren’t just team members; they have invested in every success, every customer interaction, and every innovation.
The Long-Term Investment: Growing Value Over Time
And the longer you stay, the more you gain. The ESOP serves as a retirement plan, building employees’ investments over time–no extra steps required to earn stock as an employee, just dedication to the company.
 C&J educates its team on the unique value of an ESOP, helping employees understand how their contributions impact not only the stock value and bottom line but also their personal stake in the future.
C&J educates its team on the unique value of an ESOP, helping employees understand how their contributions impact not only the stock value and bottom line but also their personal stake in the future.
This sense of ownership inspires pride in the work, the quality of the products, and the level of service each customer receives. Plus, the ESOP is a powerful recruitment tool, attracting talent excited to have a real stake in the company’s success.

A Legacy of Integrity and Shared Vision
CEO Jerry Sargent encourages other business owners to consider ESOPs, explaining, “Too many manufacturers are being sold to much larger organizations. If you’re an owner who wants to keep your vision alive and see your employees carry it forward with the same passion, the ESOP model gives them the chance to buy the company from you.”
For C&J, ESOP means a legacy built on integrity, trust, and shared ambition. Customers also see the impact, recognizing the dedication of employee-owners and rewarding C&J with their loyalty and repeat business.
Looking Ahead: Growth & Expansion
As the new year approaches, C&J’s excitement builds–they are nearing their ESOP loan payoff & putting the final touches on a major 25,000 sq ft expansion. Each year, employees eagerly review their stock values, reflecting C&J’s continued success and growth. “We want to continue to grow the company wisely,” Jerry said. “Continuing the founders’ legacy, and securing the future of all associates.”
The post Unlocking the Power of Ownership: C&J Industries’ ESOP Journey appeared first on CJ Industries.
Innovative Syringes Change the Game 7 Oct 2024, 5:37 pm
With recent advancements in pharmaceuticals, the syringe market has experienced significant changes. Traditional syringes have limitations that make them incapable of serving the entire market. The introduction of COP (cyclic olefin polymer) and COC (cyclic olefin copolymer) plastic syringes mark a progressive and necessary change in the market; this new technology will not only simplify tasks for healthcare professionals, but will also make products safer for patients.
Benefits Over Glass:
- Cost: 1/4 to 1/10 the cost of glass syringe luer lock syringe assemblies
- Shatter Resistance: Will not shut down pharma filling production line
- Weight: <50% that of glass thus reducing shipping costs
- Design Flexibility: Finger grips, luer tip style, back stop – molded in features
- Very Low Protein Absorption
Benefits Over Polypropylene (PP):
- Higher Purity
- Higher Modulus
- Glass-like Clarity
- Sterilizability
- Greater Chemical/Heat Resistance
- Higher Moisture Barrier
- Low Moisture Absorption Rate
COC features glass-like clarity while also being break-resistant, lightweight, and withstanding conventional sterilization methods. It is suitable for a range of medications, including viscous drugs, and provides for an extended shelf life. The unique processing methods allow for the production of a high quality product that exceeds the accuracy and performance standards set by glass syringes. The superior moisture barrier with COC medical syringes extends the shelf life of some pharmaceutical solutions by 3 years, a standard that can’t be met by other thermoplastics used in less challenging applications. With COC syringes, the drug remains pure because COC is biologically inert, with very low extractables.
COP plastic syringes have similar properties to COC syringes, but are even less brittle and can be made into more varied size and complex shaped containers. COP syringes are increasingly popular in applications where high viscosity products require high strength and high transparency to ensure the product is thoroughly injected.
PP (Polypropylene) films use COC as a performance enhancer to simplify films and compete with more complex, less recyclable structures. The extremely high purity and unique properties of COC have made it the dominant material in advanced diagnostic and microfluidic applications. The material’s use in primary drug packaging has become much more prevalent as newer pharmaceutical molecules and formulations trend toward more chemical sensitivity rather than lower-purity plastics and glass.
Typical COC material has a higher modulus than PP. COC also has a high moisture barrier for a clear polymer along with a low moisture absorption rate. Importantly for medical uses, COC is sterilizable by all standard methods including steam, EtO, gamma, and hydrogen peroxide. The optical properties of COC are exceptional, and in many ways very similar to glass. COC materials offer exceptional transparency with a high chemical and heat resistance. Low moisture absorption rates of COC are often an advantage over competing materials such as polycarbonate and acrylics.
Overall, the benefits of COC (cyclic olefin copolymer) plastic syringes and its chemical cousin COP (cyclic olefin polymer) are unmatched. When compared to traditional syringes, this new material provides lower cost options, better breakage resistance, lighter weight, more design flexibility, higher purity, higher modulus, and less absorption of the drug, just to name a few. These types of advancements demonstrate a promising future for the medical and pharmaceutical industry, which is always striving to not only make life easier for healthcare professionals, but also to make products safer for patients.
Sources:
- Sparrow N. Pharma company sees clear advantages in COC syringe. Plastics Today. November 16, 2023. Accessed August 29, 2024. https://www.plasticstoday.com/medical/pharma-company-sees-clear-advantages-in-coc-syringe.
- Equinox Syringes. COP and COC Prefillable Syringes. Plastechengineering. 2024. Accessed August 29, 2024. https://www.plastechengineering.com/glass-replacement.
- Kneale T. Medical plastics 101: Cyclic olefin copolymer. Plastics Today. July 11, 2024. Accessed August 29, 2024. https://www.plasticstoday.com/medical/medical-plastics-101-cyclic-olefin-copolymer-fulfills-complex-medtech-performance-requirements.
The post Innovative Syringes Change the Game appeared first on CJ Industries.
C&J Industries Expands Cleanroom Capacity and Gains Insights from ESTECH 2024 12 Sep 2024, 3:50 pm
In case you missed it, C&J Industries is undergoing a significant facility expansion, which includes the addition of a new cleanroom. This expansion is set to nearly double our Class 8 Cleanroom medical injection molding capacity, in addition to the cleanroom molding we perform cleanroom assembly operations. This will enhance our capabilities in molding and assembling high-quality medical devices and other critical components.

To ensure top-notch cleanroom protocols and training, we recently sent Cameron Costner, Regulatory Affairs Administrator, to the Institute of Environmental Sciences & Technology’s ESTECH Conference in Las Vegas, Nevada. ESTECH is the leading conference for professionals in the fields of contamination control, cleanrooms, controlled environments, product reliability, environmental testing, and nanotechnology. Cameron shared his experiences and insights from the conference, shedding light on the importance of maintaining and improving cleanroom standards.
A Dive into ESTECH 2024
Cameron described his attendance at ESTECH 2024 as “extremely valuable.” The conference, filled with experts ranging from microbiologists to cleanroom designers, provided a rich learning environment. Cameron participated in various sessions, including a boot camp-style training that covered the design, build, and testing of cleanrooms.
 “The first few days were geared around the design, build, and testing for the cleanroom—kind of like the ins and outs of how you should design it. As far as where you should have your diffusers, your air returns, and how you determine particle count locations,” Cameron explained. This foundational knowledge is crucial for ensuring compliance with the ISO 14644 standard, which governs cleanroom classifications and air cleanliness levels.
“The first few days were geared around the design, build, and testing for the cleanroom—kind of like the ins and outs of how you should design it. As far as where you should have your diffusers, your air returns, and how you determine particle count locations,” Cameron explained. This foundational knowledge is crucial for ensuring compliance with the ISO 14644 standard, which governs cleanroom classifications and air cleanliness levels.
Another significant aspect of the training was the emphasis on cleaning protocols. “The second big class that I attended was around cleaning and the importance of ensuring the cleanroom areas and workstations are clean. Not just visibly clean, but making sure we’re eliminating the bacteria,” Cameron highlighted. Understanding best practices for cleaning frequencies and the appropriate garments to wear in the cleanroom are vital for maintaining a contamination-free environment.
Integration into C&J Industries
Cameron reassured that the new cleanroom will be seamlessly integrated into C&J’s existing processes. “We’re working to integrate the new cleanroom into our current training regimen,” he said. This involves updating documentation and forms specific to the new cleanroom while maintaining existing standards.
In order to keep up with ISO & FDA standards, cleanroom training at C&J is ever-evolving. Cameron believes his experience at ESTECH will help inform C&J’s training efforts in the new cleanroom. “At ESTECH, I was able to reaffirm that what we’re doing in the C&J cleanrooms is in compliance with those standards, but I also gained a better understanding of the cleanrooms themselves and how best to train our cleanroom teams,” Cameron elaborated. “It’s important that we cross-train everyone so they all understand the protocols and can run every job.” This approach ensures that employees are versatile and capable of operating in different environments, whether it’s a cleanroom or a less controlled white room.
Rigorous Testing and Quality Assurance
Ensuring that the new cleanroom meets all standards involves several testing phases. This thorough testing ensures that the cleanroom functions correctly under various conditions and maintains the necessary air quality and particle count standards.
C&J Industries is also responsible for monitoring the cleanrooms’ particle counts and air change rate. “We do an annual qualification to ensure the cleanrooms meet the criteria for ISO classification,” Cameron explained. “And as part of our continuous monitoring program we take a particle count at set locations and daily air pressure checks to ensure that we meet the ISO requirements.”

Looking Ahead: The Future of Cleanrooms at C&J
Progress continues to be made on the expansion, including the cleanroom and a tunnel to connect our two facilities. Cameron emphasized the long-standing expertise of C&J in cleanroom work.
“C&J has been doing cleanroom work for a long time. Luckily for me, when I came into this position, we’d been doing cleanrooms for 20-plus years already, so I inherited a great system,” Cameron said. “I’m just trying to continue to learn and improve our cleanrooms and our processes and ensure that we’re doing it right, being compliant, and making great products for our customers.”
Cameron’s attendance at ESTECH 2024 and the ongoing efforts at C&J highlight the company’s commitment to excellence in cleanroom standards and operations. As C&J Industries continues to expand and innovate, our dedication to maintaining high-quality cleanroom environments in the field of medical device manufacturing will remain unwavering.
Follow our journey as we expand and enhance our cleanroom capabilities. Discover how our commitment to top-notch standards in medical device manufacturing can benefit you. Connect with us.
The post C&J Industries Expands Cleanroom Capacity and Gains Insights from ESTECH 2024 appeared first on CJ Industries.
The Heart of C&J Industries’ Customer Service 14 Aug 2024, 7:34 pm
At C&J Industries, exceptional customer service has a name: Tommy. As Customer Liaison, Tommy Saulsbery has built a stellar reputation, earning respect and admiration from everyone he works with. It’s no wonder everyone loves Tommy!
Tommy has been a part of the C&J team since August 2008. He lives in Meadville with his wife and their two boys; aged 4 years old and 8 months. Starting at C&J right out of high school, Tommy has thrived in his current role for almost 8 years. “I really enjoy working with the customers,” Tommy said. He’s learned that at C&J, both the quality of your work and your work ethic are recognized and valued.
What exactly does a Customer Liaison do? According to Tommy, they handle customer orders from start to finish. Beginning with entering orders into the ERP (enterprise resource planning) system and then ensuring they are shipped to the customer. Acting as a crucial link between customers and various departments, they also prepare all necessary documents for overseas shipments.
While the customer service department has seen steady growth in line with C&J’s expanding customer base, its core remains unchanged. Tommy explained, “If I can’t answer the question immediately, I will ask the applicable department, and then get back to the customer.” He takes great pride in providing prompt answers, always striving to assist customers as efficiently as possible.
Tommy doesn’t shy away from challenges, tackling problems head-on. When sourcing resin became particularly difficult, Tommy quickly identified where the resin was used and reallocated it effectively. His proactive and hands-on approach has been crucial in resolving such issues efficiently.
Tommy believes that what sets C&J’s customer service apart is their unwavering commitment to communication and responsiveness. “At C&J, we excel at maintaining open lines of communication and providing prompt responses to our customers,” Tommy says. “We understand the importance of addressing concerns quickly and producing emergency orders as fast as possible. This dedication to meeting our customers’ needs is what truly sets us apart and drives our success.”
Helping customers in any way is the name of the game, and at the heart of our customer service success is Tommy Saulsbery. He exemplifies dedication and problem-solving, ensuring customer satisfaction. We’re incredibly grateful to have Tommy on our team, and we look forward to seeing what the future holds for him and C&J customer service. If together we are as successful as Tommy is loved, there are no heights we can’t reach!
If you’d like to speak to Tommy or know more about how C&J Industries can help you, please contact us.
The post The Heart of C&J Industries’ Customer Service appeared first on CJ Industries.
Flexibility, Versatility and Efficiency Leading the Way 7 May 2024, 1:25 pm
As tools improve, processes and productivity inevitably follow suit. This principle has guided manufacturers across various industries and countless years, and the modern wave of automation is no exception.
To that end, Meadville, PA-based specialty assembly and injection molding firm C&J Industries is in the midst of an expansive and ambitious effort to implement cobots (or collaborative robots) into its established workflow, with the intended effect of supplementing the existing human workforce.
With any implementation of new technology – especially those that bring about adjustments to workflow – some growing pains are inevitable. However, so far, the push for automation at C&J has proceeded with minimal friction according to automation engineer Jeremy Slagle.
“I think it was a pretty smooth transition,” Slagle said. “Regarding all these various parts of the product stream and all the interruptions that introducing this new part of the process can cause … I think the whole team, everyone involved at the production level at C&J are very accommodating to these changes, especially the ones that help us.”
One factor that has been key to C&J’s successful integration of automation technology is the company’s overarching goal of leveraging the new equipment to support members of the assembly teams without making their positions obsolete.

“(The cobots are used) not as a replacement, but as a helpful tool for our operators to use that reduces the workload on them and opens up more time for them to do things that aren’t those menial tasks,” Slagle said. “For example, one robot works on a printing process that previously took two or three operators, and now it’s down to one operator, who essentially hands off the molded part to the robot and the robot takes it and handles all the printing operations, then essentially hands it back once the part’s all done in a timely fashion.”
In this way, the cobot functions as a programmable partner capable of complex tasks, boosting the efficiency of the entire workflow.
“It works out nicely for that operator to have a comfortable pace, hand off these parts to a robot, and when they receive them back, they have time to inspect it, package it, and move on to the next part,” Slagle added. “They’re very nicely timed with each other. And our operators really like using the robots. That’s sort of the angle I’ve been keeping with the implementation – they’re not a replacement for anybody, they’re there to use and help them do their jobs easier.”
As the C&J staff become more comfortable working alongside cobots and other automated equipment, Slagle said the goal is to apply these new processes to a broader scope of projects for a greater variety of clients.
“When we made our initial investment in our first cobot setup, we went with a very modular mindset where we got all the bits and pieces to be able to do a lot of different jobs with it,” Slagle said. “We’re making the investment in ourselves to be proficient in setting these things up ourselves and being able to move them around the plant efficiently. We’re still moving them around and trying different things. The big emphasis is on flexibility.”

Cobots built to operate alongside and under the close supervision of human technicians are only the beginning. Innovations are routinely being applied to these devices; including advanced methods of autonomy such as camera-enabled vision guidance.
“Not only did we invest in the six-axis robot, but we also invested in control systems, two different vision systems, getting into vision-guided robotics – 2D and 3D – where the robot essentially has the vision to drive itself,” Slagle said. “One of the new technologies we’ve been working on is bin-picking vision, which isn’t really new to the industry but it’s pretty new to us, to where a camera mounted on a gantry above a box of parts can take a picture and then it tells the robot how to go get it without you having to actually program all those points.”
As C&J continues to invest in innovation, the company also continues to invest in its employee-owners. Slagle, having begun his career with C&J as an intern in 2014 during his education at Penn State Behrend, is a prime example of this investment. Since his recent promotion from manufacturing engineer to automation engineer, he has taken on greater responsibility and a more personal perspective on the company’s evolution.
“It’s nice that the company where I started at, did a lot of early learning at, and going from my learning phase to now – I really feel like I’m a part of our growth. I’m sharing the responsibility and just feeling like I’m a part of the company that’s really helping it grow at this point.”
Looking ahead, Slagle hopes to “stay the course” by continuing to take an active role in C&J’s automation journey and ongoing expansion.
“The level of automation we were at 10 years ago versus now is very different, and more and more of it’s coming in all the time. It just feels like we’re on a good path and I’m just here to keep us moving in this direction and help us keep growing.”
For more information about automation at C&J Industries, or if you have questions about how we’re using cobots, please contact us.
The post Flexibility, Versatility and Efficiency Leading the Way appeared first on CJ Industries.
C&J’s Comprehensive Approach to Project Engineering 4 Apr 2024, 8:29 pm
A well-tuned machine relies on every one of its components to operate efficiently. In much the same way, a manufacturer relies upon its constituent departments to work in seamless unison.
With efficiency and low-friction processes serving as overarching goals, C&J Industries – a specialty assembly and injection molding firm based in Meadville, PA – has made a concerted effort to bring its myriad resources and areas of expertise into a synchronized operation. Additionally, in the wake of a recent 25,000 sq. ft. facility expansion, C&J is uniquely positioned to put its time-tested processes to use for bigger, more complex client projects.
Engineering manager Jordan Walker, who oversees the design engineering team at C&J, believes one of the company’s greatest strengths is the wealth of experience and knowledge – from tool design and implementation to quality inspection – that can be leveraged across the breadth of the company to address any challenge.
“We’ve got a very strong, cross-functional team and we’ve got experience throughout each of the phases of the process,” Walker said. “We are experts in all disciplines, but not each person is an expert at everything. Within our team here, we seem to have a very good feel for each individual area and we work as a team up here as needed. So, when we have a challenge with an inspection process, we rely on our in-house expert to help us along.”
A solution is never far away
Walker and his team pride themselves on their ability to troubleshoot any production stoppage or error that prevents a client’s project from moving forward. One tool in the arsenal that has helped to sharpen C&J’s engineering processes is the effective use of simulation. While simulation engineering is not unique to C&J, the fact that Walker and his colleagues have in-house simulation resources – as opposed to requiring an outside provider – is a clear advantage.
“When we design a tool, we actually go through a simulation process so that we’re able to predict what is going to be the output of that tool,” Walker said. “We also use it in reverse; if we have a challenge down on the floor, we can run it through simulation, verify that what we’re seeing is representative of the real tool, then we can try some modifications and re-run the simulation. It’s a tool that allows us to basically anticipate what the real-life output’s going to be, prior to completing the design.
“Having it in-house is definitely an advantage for us, as some (workshops) may need to go to a software house or simulation house that they outsource to,” he added.
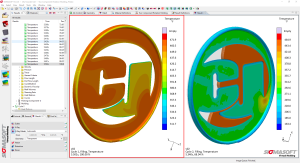
For the engineers at C&J, ensuring the success of a partner’s project can start even before the simulation phase. Walker finds that a collaborative design process between his team and the client can make all the difference.
“Project-wise, we’ve been able to work with design concepts with the customer; structurally designing something, understanding what their need is,” Walker said. “A larger customer of ours – they’ve been around for 20 years plus – essentially, they were just looking for a structurally sound part. We were able to run some design iterations and figure out where they needed to be in order to meet that standard.
“One of the clean rooms we actually have in-house here was built to enable our customer to get to market quickly and that was managed through one of our project engineers working in turn with our (design engineering) team to make sure we were able to coordinate all the efforts to come together at the right time,” Walker added. “We were building a piece of semi-automation equipment, we were installing a room and doing a room layout, and making sure we had it all together at the same time so that we could help them get to market.”
Proven processes that deliver on time
Heading into the new year, Walker hopes that the various departments that comprise C&J will continue to fine-tune their processes and get the overall organization thinking about how to apply simulation engineering and other cutting-edge resources to their projects.
“We’re continuing to evaluate our processes and look for efficiencies and making sure that communication is there,” Walker said. “We’ve used simulation in this building for 15 years, but I’m not sure it’s getting the visibility that it can within our company. So, that’s one of the focus areas; get the visibility, get the awareness and see it utilized better across the company so that it can be implemented and leveraged more.”
Walker also plans to put an even greater spotlight on the quality and variety of engineering skills his department can bring to bear – a strength he thinks will continue to serve C&J well in the new year.
“One thing we pride ourselves on is that, historically, we’ve been heavy on the engineering side across the company. That is one of the focuses I think we’ve had over the years: the engineering support you can get at C&J. The system base that we have here has been very effective over the years. Obviously, there have been improvements over time, but we have a process that steps through each phase to make sure that we’re successful at the end of that process and that we’re able to launch production reliably.”
If you want more information or would like to explore opportunities to boost your project engineering, please contact us.
The post C&J’s Comprehensive Approach to Project Engineering appeared first on CJ Industries.
Focusing on Growth in 2024 9 Feb 2024, 3:24 pm
Invigorated by a recent expansion, C&J Industries aims to aggressively pursue new customers in the new year.
With new capabilities come new opportunities, and new capabilities are exactly what Meadville, PA-based specialty assembly and injection molding firm C&J Industries has been doing. As they look ahead to 2024, the company’s employee-owners look to capitalize on their expanded capacity and improved equipment.
During the third and fourth quarters of 2023, C&J renovated its plant with a 25,000-square-foot addition, including a new clean room. The firm has also invested heavily in automation solutions to streamline operations and allow for the execution of larger, more complex projects. These factors all add up to a company invigorated with new energy and the capacity to grow even further.
In light of the expansion, Regional Sales Manager Barry Stainbrook is looking forward to showcasing C&J’s new-and-improved facility to existing and potential clients.
“With this expansion, we’re going to be able to walk someone on the floor and say ‘we have an extra 5-10,000 feet here that we can offer you for your project.’ I think that will be a big selling point,” Stainbrook said. “Any time we’re expanding clean room capabilities, that’s a big deal too, because that is something medical manufacturers are looking for.”
A one-stop shop with local roots
The strong points that set C&J apart in the injection molding and specialty manufacturing spaces go beyond having the right equipment and expertise. Stainbrook, who was a toolmaker by trade before going into sales, personally values C&J’s dedication to utilizing local supply lines and subcontractors as much as possible – something customers value, as well.
“I love having everything here. I love not going offshore – not that we haven’t in the past when we needed to, but once we were dealing with COVID and all the deliveries and tariffs and things, we really pushed domestic (operations),” Stainbrook said. “Even if we don’t build it here, we still try to build domestic, and that can be local through sub-contractors. But being able to control the design, the build, the qualification, everything here – that’s definitely a big deal for customers.”
With integrated tooling and fabrication support on-site, C&J project engineers can reliably deliver for their clients with the knowledge that parts can be repaired or replaced as needed, without needing to heavily impact the client’s timeline.
“If (a customer) just goes to a molding provider that doesn’t have that tooling support, they’re going t

o pull that tool out, send it back to whoever built it, bring it back in again and load it, whereas we might be able to do something in a day,” Stainbrook added. “We can pull the mold over to the mold maintenance area and work on it. So, that’s a huge advantage when it comes to saving time.”
This flexibility is aided by C&J’s connections to industry partners throughout Pennsylvania and the surrounding region. Such roots in the community mean that even if C&J engineers don’t have a solution in-house, they know where to find one nearby.
“Local to us, within a few miles; we have heat treating here, we have plating for these tools, we can even do some welding here but we also have a welding shop in town. So, our subcontractor base is very strong around here,” Stainbrook said. “And that could also mean that for components or maybe a large mold base that might go to Erie (Pennsylvania) to get finished, we’ll do the interior.
Building a Smarter Warranty
Inevitably, things break. When they do, it pays to have a plan in place to replace or repair the affected component. Manufacturer warranties are nothing new, but C&J attempts to take an adaptable and transparent approach with its 10-year mold warranty, which covers any production tool operating within the C&J facility.
According to Stainbrook, the warranty replaces or repairs any covered machinery for up to ten years or for a predetermined number of parts fabricated – whichever comes first. The warranty is supported further by C&J’s in-house engineering support, capable of servicing warrantied parts on-site. The result is a safety net that keeps client projects on schedule without subjecting the customer to nickel-and-dime expenses.
“If you don’t have a warranty on a tool, and say it does go down and breaks something; now we have to quote it, go out to the customer, get the purchase order – and that can take a while – and then get back into production,” he said. “Whereas if it’s under us, we either have spares here ready for it that we can just change out and put in, or we just know we need to make them and we go right from there. It cuts weeks out of that tool being down, so that’s a big advantage.
“At the end of that mold warranty, we evaluate that tool and reach back out to the customer and say ‘we can put another warranty on this tool but we’re going to want to refresh it and bring it back up to where it originally was,’” Stainbrook added.
Although pursuing new business has always been a goal for C&J, the new year will see a renewed focus on prospecting thanks to the recent expansion, with target markets including the medical and telecom industries. Between the local resources at their disposal and other selling points such as the 10-year mold warranty, Stainbrook is confident that the company can go into 2024 on the right foot.
The post Focusing on Growth in 2024 appeared first on CJ Industries.
C&J Industries Receives ISO 13485 & ISO 9001 Recertification 8 Dec 2023, 7:38 pm
We are proud to announce that after an external audit, the Quality Management System of C&J Industries, Inc. has been assessed and approved by DQS, Inc., and fulfills the requirements of the ISO 13485:2016 and ISO 9001:2015 standards.
These re-certifications, completed December 2023, each consist of a complete certification audit every 3 years with yearly surveillance audits, to verify that the C&J Industries’ Quality Management System continues to meet the requirements for ISO 13485 and ISO 9001.
Since 2009, C&J Industries has partnered with DQS, an accredited certification body, to achieve these ISO certifications.
“These certifications reflect our long-standing commitment to maintain high levels of quality and reliability to meet or exceed our customers’ expectations,” said Cameron Costner, Regulatory Affairs Administrator at C&J Industries. “We will continue to look for opportunities to improve our processes and ensure our Quality Management System remains effective.”
ISO 13485 is the globally recognized standard for organizations involved in the design, production, installation and servicing of medical devices and related services.
ISO 9001 is a globally recognized standard for quality management. It helps organizations of all sizes and sectors to improve their performance, meet customer expectations and demonstrate their commitment to quality.
Learn more about our quality policies and view our certificates here.
The post C&J Industries Receives ISO 13485 & ISO 9001 Recertification appeared first on CJ Industries.
Expanded facility, greater automation in the future for C&J Industries 21 Nov 2023, 4:17 pm
The Meadville, PA manufacturer is investing in its growing assembly business with a new clean room, collaborative robotics and more.
For more than 61 years, C&J Industries has been an innovator in the injection molding and specialty assembly spaces. Even the turbulent post-COVID period couldn’t slow this march, as the company’s employee-owners continue to invest in their ability to fulfill large and complicated orders for customers.
These customers include some of the leading organizations in the healthcare and telecom industries, all of whom rely on C&J’s expertise and dedication to quality.
Soon, the company will be in a position to expand its loyal customer base thanks to a larger facility footprint and updates to the equipment within. The 214,000-square-foot facility is currently being renovated with a 25,000-square-foot addition, including a new “clean room,” adding more molding and assembly capacity.
“The building itself is being expanded, and that is primarily for a new clean room that is being built to handle some incoming jobs from existing customers, as well as to help provide molded components for a new job that we’re getting in Assembly,” said assembly supervisor John Beers. “We have a clean room that is being expanded for a new job that’s in assembly, and that will be a fully automated piece of equipment, which is pretty exciting, actually.”
When completed, the facility upgrades will allow C&J to increase production volume, take on jobs of a larger scope, and expand services to new clients. This development reflects the company’s efforts to rebuild its staffing and capabilities to pre-pandemic levels.

Although the COVID outbreak in 2020 had an unavoidable impact on C&J’s supply lines, the company was able to survive the pandemic without resorting to layoffs.
“We didn’t consciously cut back, necessarily, during COVID. In fact, we were probably one of a few businesses that didn’t. We lost people just as they went to different places, and we had some folks decide to retire … but we didn’t lay anybody off through any of that, which is a really great thing for us,” Beers said. “Overall, we had a big hiring boost shortly thereafter, we brought levels up to a certain point, and now we’ve kind of backed off a little bit and we’re right about where we were, I would say, pre-COVID.”
Now on the other side of these staffing challenges, C&J has learned some valuable lessons on how automation can maximize efficiency by complementing the human workforce.
“While we were dealing with some of the labor issues, we looked at more efficient processes. We were able to transition (some things) – say, we used a pad printing process to print different components before we assembled them, and it was very labor-intensive,” Beers said. “Fortunately, the customer we dealt with for that project purchased a different type of equipment and allowed us to merge that printing process into the assembly stage at the same time and saved a lot of that labor.
“So, even though we didn’t cut anybody out, we were now able to redirect those people to different jobs,” he added. “We had roughly the same number of people, but we were able to do more with that because we’re more efficient.”
Another aspect of C&J’s ongoing evolution is the implementation of cobots (or collaborative robots) in its operations. Designed to be used in close proximity with human operators, these machines can be used to turn a two-person job into a one-person job, with the overall goal to “continue meeting the expectations” of clients and “exceed them where we’re able to,” according to Beers.
Out of a crowded field of injection molding and assembly specialists, C&J’s clients continue to choose them. Beers believes one possible reason for that is the mutual interests and bonds of professional respect that tie C&J and their customers together.
“We seek out customers that want us to succeed and we want them to succeed in return. A lot of times, we’ll have customers that will help us invest in new technologies and we may even use customer-owned assets,” Beers said. “Also, there’s the fact that we’ve remained local. With the last president that we had, when he retired, instead of selling to somebody else, he allowed us to move forward 100% employee-owned.”
As employee-owners, the team at C&J has skin in the game and tangible investment in the company’s success. This makes a clear difference to their clients, who value knowing that they’re dealing directly with the manufacturers themselves.
“We’ve seen a lot of businesses in the Meadville area get purchased and sold over and over again, and different things that upset the balance; so I respect the fact that the people in charge wanted to make sure we stayed local,” Beers said. “That way, the employees themselves are invested at that point, because it impacts the community and their friends and coworkers.”
The post Expanded facility, greater automation in the future for C&J Industries appeared first on CJ Industries.
ISO Certifications: 9001 vs. 13485 and Their Benefits 15 Jun 2023, 6:42 pm
ISO (International Organization for Standardization) is an independent, non-governmental organization which certifies that a management system, manufacturing process, service, or documentation procedure has all the requirements for standardization and quality assurance. Companies that are ISO certified experience an improved level of quality with services, processes, and products. C&J Industries is ISO:9001 and ISO:13485 certified.
ISO 9001 is an internationally recognized standard that sets the requirements for a quality management system (QMS) that is centered on the idea of continual improvement. This standard focuses on enhancing customer satisfaction by meeting customer requirements and delivering consistent, high-quality products and services. By implementing ISO 9001, organizations can improve operational efficiency, enhance customer satisfaction, reduce waste and errors, and drive continual improvement across all areas.
Here are key aspects of ISO 9001:
- Customer Focus
- Process Approach
- Risk-Based Thinking
- Continual Improvement
- Documentation and Control
- Management Responsibility
- Employee Involvement
- External Recognition
ISO 13485 is an internationally known standard for quality management system (QMS) which solely applies to the manufacture of medical devices. This standard sets out the requirements for organizations involved in the design, development, production, installation, and servicing of medical devices. ISO 13485 focuses on ensuring the safety, reliability, and effectiveness of medical devices and promoting compliance.
Here are key factors of ISO 13485:
- Regulatory Compliance
- Risk Management
- Product Lifecycle Management
- Documentation and Record Keeping
- Customer Focus and Feedback
- Management Responsibility
- Supplier Management
- Continual Improvement
Obtaining ISO certifications offers many benefits such as:
- Enhanced Credibility and Reputation: ISO certifications are globally recognized and are a commitment to quality, efficiency, and continuous improvement.
- Improved Quality Management: ISO certifications, such as ISO 9001, focus on establishing and maintaining effective quality management processes. This leads to improved customer satisfaction and increased confidence in the organization’s ability to deliver reliable and high-quality outcomes.
- Compliance with Regulatory Requirements: Achieving ISO certifications ensures that an organization meets the necessary compliance standards, demonstrating obedience to industry-specific regulations and legal obligations. This can simplify regulatory audits, facilitate market access, and enhance overall compliance practices.
- Operational Efficiency and Cost Savings: ISO certifications emphasize the adoption of efficient processes, waste reductions, and continuous improvement. This leads to cost savings, improved productivity, and optimized resource utilization.
- Increased Customer Satisfaction: ISO certifications, particularly ISO 9001, focus on meeting customer expectations and enhancing satisfaction. By implementing customer-centric processes and quality management systems, organizations can improve their ability to deliver products and services that meet or exceed customer requirements. This leads to customer satisfaction, loyalty, and positive word-of-mouth referrals.
Here is how ISO certifications can benefit the customer:
- Assurance of Quality: ISO certifications, such as ISO 9001, provide customers with assurance that a robust quality management process has been implemented. Organizations can demonstrate their commitment to delivering products and services that meet or exceed customer expectations. Customers can trust that the organization has implemented quality controls, processes, and procedures to ensure consistent quality and reliable outcomes.
- Consistency and Reliability: The adherence to ISO standards means that the organizations follow established protocols and practices to achieve consistent quality across its offerings. Customers can rely on the fact that their experience will be consistent and meet the same high-quality standards ever time.
- Customer Focus: Organizations with ISO certifications have systems in place to monitor and measure customer satisfaction and to take appropriate actions for continuous improvement. This approach means that organizations are more likely to respond to customer needs, preferences, and feedback for an overall better customer experience.
- Confidence in Supplier Selection: By selecting suppliers with ISO certifications, customers can have confidence in the supplier’s ability to consistently deliver quality products or services, reducing the risk of non-compliance or subpar performance.
- Effective Problem Resolution: Organizations with ISO certifications are equipped with procedures and mechanisms to identify, address, and resolve customer complaints or issues in a timely and effective manner. This ensures that customer concerns are considered.
- Increased Transparency and Accountability: Customers benefit from increased transparency in how the organization operates, as well as clear lines of responsibility for quality management. This transparency and accountability contribute to the customer’s understanding of the organization’s commitment to quality and its ability to deliver on its promises.
- Reduced Risk and Liability: Choosing certified organizations allows customers to reduce the risk of receiving substandard products, facing quality issues, or experiencing disruptions due to poor quality management issues. This reduces potential liabilities and costs associated with rework, returns, or non-compliance with quality requirements.
In conclusion, organizations choose ISO 9001 and ISO 13485 certifications to enhance their quality management practices, meet customer expectations, comply with regulations, improve operations efficiency, mitigate risks, and gain a competitive edge in their respective industry. ISO certifications provide customers with confidence in the quality, consistency, and reliability of products and services offered by certified organizations.
Here at C&J Industries, we have been proudly ISO 9001 certified since March 3, 1994, and ISO 13485 since December 22, 2009, which reflects our compliance with recognized standards, demonstrating our commitment to maintaining the highest levels of quality, reliability, and compliance. Our ISO certifications drive our commitment to continuous improvement. In our facility, we have one (1) Class 7 Cleanroom and five (5) Class 8 Cleanrooms that are internally certified to the ISO 14644 standards which is the classification of air cleanliness and is associated with controlled environments.
The post ISO Certifications: 9001 vs. 13485 and Their Benefits appeared first on CJ Industries.