Add your feed to SetSticker.com! Promote your sites and attract more customers. It costs only 100 EUROS per YEAR.
Pleasant surprises on every page! Discover new articles, displayed randomly throughout the site. Interesting content, always a click away
Pharmaceutical Processing World
The Leading Source for Pharmaceutical & Biopharmaceutical Manufacturing NewsNovartis to expand U.S. manufacturing, R&D footprint with $23B investment 10 Apr 2025, 7:06 pm
 Novartis announced today that it plans to invest $23 billion over five years to expand its U.S.-based infrastructure.
Novartis announced today that it plans to invest $23 billion over five years to expand its U.S.-based infrastructure.
The company said its investment aims to ensure all key Novartis medicines for U.S. patients will be made in the U.S. Its financial commitment enables the company to expand on its current pharmaceutical manufacturing, research and technology presence across the company. This includes 10 facilities, with seven of them brand new, creating nearly 1,000 jobs at Novartis and about 4,000 additional U.S. jobs. Production capacity covers both active pharmaceutical ingredients (API) and biologics drug substance, plus secondary production and packaging.
Novartis plans to establish a $1.1 billion biomedical research innovation hub in San Diego, creating its second global R&D hub in the U.S. The company expects to complete this complex between 2028 and 2029. Serving as the epicenter of the Novartis West Coast Biomedical Research presence, it complements existing hubs in Cambridge, Massachusetts and Basel, Switzerland.
It also plans to build four new manufacturing facilities in soon-to-be-determined states. Three of those will make biologics drug substances, drug products, device assembly and packaging. The company plans for the remaining facility to make chemical drug substances, oral solids dosage forms and packaging.
Other plans include the building of two new radioligand therapy (RLT) manufacturing facilities in Florida and Texas. Novartis also intends to expand three RLT manufacturing plants in Indianapolis, Millburn, New Jersey, and Carlsbad, California.
With its investments, Novartis said its manufacturing capacity in the U.S. can cater to all core technology platforms. It brings its internal siRNA technology manufacturing to the U.S. for the first time, too.
“As a Swiss-based company with a significant presence in the US, these investments will enable us to fully bring our supply chain and key technology platforms into the US to support our strong US growth outlook. These investments also reflect the pro-innovation policy and regulatory environment in the US that supports our ability to find the next medical breakthroughs for patients,” said Vas Narasimhan, CEO of Novartis. “We are prepared for shifts in the external environment and fully confident in our 2025 guidance, mid- to long-term sales growth outlook and 2027 core margin guidance of 40%+.”
The post Novartis to expand U.S. manufacturing, R&D footprint with $23B investment appeared first on Pharmaceutical Processing World.
BDD Pharma opens new GMP manufacturing plant 8 Apr 2025, 7:25 pm

[Image courtesy of BDD]
The company opened its facility after two years of development following a successful inspection by the Medicines and Healthcare products Regulatory Authority (MHRA) last month.
A £2 million funding round from existing investors facilitated the development of the state-of-the-art facility. Investors included leader Archangels, as well as Scottish Enterprise and new investor British Business Bank.
The GMP facility, constructed by cleanroom specialists Guardtech Group, expands BDD’s manufacturing capabilities and clinical testing services. It supports R&D for small and medium-sized pharmaceutical companies, according to a news release.
Stephen Clarke, head of quality at BDD, said: “This new additional facility provides a wonderful opportunity to generate even quicker reliable data on the safety and effectiveness of these newly developed medicines. That in turn will lead to new medicines getting into patients quicker and so improve patient health sooner, which benefits all of society.
“The outcome of the recent UK Government Inspection provided a high level of independent confidence that we are doing all the correct things, in the correct way, consistently.”
Sarah Hardy, Director and Head of New Investments at Archangels, said the investment in the new facility strengthens BDDs ability to offer end-to-end services to meet the growing market demand.
“BDD’s uniquely supports pharmaceutical companies of any size that need to act quickly or accelerate plans enabling them to navigate the complex path of getting new therapies to patients,” Hardy added.
The post BDD Pharma opens new GMP manufacturing plant appeared first on Pharmaceutical Processing World.
Ashland expands pharmaceutical tablet coating plant in Brazil 3 Apr 2025, 8:00 pm
 Ashland announced that it completed a $10 million expansion of its pharmaceutical manufacturing plant in Cabreúva, Brazil.
Ashland announced that it completed a $10 million expansion of its pharmaceutical manufacturing plant in Cabreúva, Brazil.
The company plans to mark the occasion with a ribbon-cutting ceremony tomorrow, April 4. This investment significantly expands Ashland’s pharmaceutical footprint in the region and capabilities to meet increasing market demand for coated tablets in Latin America and Brazil.
Ashland said its investment also includes the modernization of equipment for microbial protection in personal care applications at the R&D laboratory of the company’s technical center in São Paulo. It follows previous investments to expand bioresorbable polymer manufacturing in Mullingar, Ireland, and biofunctionals R&D in Shanghai, China.
The new state-of-the-art equipment enhances Ashland’s capabilities in tablet coatings application, color matching, stability and quality evaluation. Through enhancements to the R&D lab, Ashland can offer customized solutions to industry needs, plus innovative solutions with ultra-high solids coating, moisture protection, odor and flavor masking and modified release systems. To ensure optimal performance, local Ashland employees underwent specialized training at the company’s headquarters in the U.S. and with equipment manufacturers.
“Our focused actions continue to demonstrate Ashland’s strategies to globalize, innovate and invest as a means of driving superior differentiation for customers and increase shareholder value,” said Guillermo Novo, chair and CEO, Ashland. “The investments we have made in Cabreúva and São Paulo are supporting the globalization of our expertise in tablet coatings and microbial protection. They ensure even more innovation, collaboration and technical support for Ashland customers.”
The post Ashland expands pharmaceutical tablet coating plant in Brazil appeared first on Pharmaceutical Processing World.
Seamless scaling: The role of smart automation in CGT manufacturing 2 Apr 2025, 3:49 pm
Cell and gene therapy teams face unique challenges in scaling production while maintaining precision and compliance. Strategic automation enables seamless technology transfer, integration, and scalability—turning solutions to reduce complexity into a competitive advantage.

[Image licensed from Adobe Stock]
Much of the change comes from a shift in the paradigm of treatment delivery. Today’s organizations are far more focused on identifying the specific needs of individual patients, evidenced by a shift from development of blockbuster treatments to more targeted therapeutic research over the last decade.
Cell and gene therapy takes that step to an even more granular level—if a life sciences company can better understand a patient’s or patient population’s genetics, the organization can better develop treatments that fit the needs of those patients. Moreover, they can also more efficiently and effectively tweak those solutions over time to continue delivering the best treatments possible.
As more organizations explore cell and gene therapies, they are discovering that production in this field requires new strategies. Flexibility, integration, and seamless technology transfer and scalability are all critical differentiators for success, but also particularly complex to capture at the small scale of most cell and gene therapy operations. Automation is often the solution, but teams must be strategic in the way they pursue an automation strategy.
Change creates challenges
At the heart of the challenge is the fact that cell and gene therapy development processes are often continually in a state of change. Teams are constantly adjusting processes, as well as adding software and equipment to improve operations.
For example, as a process evolves and moves through commercialization, the team might require a library information management system, process knowledge management (PKM) software, quality systems, a manufacturing execution system, and more. For each solution, there will be many options from a wide variety of suppliers. However, if the selection is not made thoughtfully, teams can quickly end up with operators needing to navigate several disparate systems, jumping back and forth among them.
Moreover, if teams do not plan well, data management can quickly become a problem. In the earliest phases of development, scientists need to make changes to the process very quickly. As the team adjusts recipes and processes, they will need to track those changes.
Today, much of that recording is done manually, often on electronic spreadsheets or electronic notebooks, but sometimes even on paper. Manually tracking adjustments, even on these electronic systems, is not only time consuming, but also prone to error.
Equipment changes also add to the complexity of cell and gene therapies. If one chromatography step does not perform adequately for a particular process, fixing the problem is rarely as simple as pulling that column out and replacing it with a new one. Often, the team will need to go back and rebuild the process around the specifications and connectivity requirements of the new equipment.
Setbacks at scale
As with any innovation, most new cell and gene therapy treatments start small as their developers work out the processes and procedures necessary to deliver the best product possible. A small cell and gene therapy site might have one bioreactor today, but the plan is never to operate that single bioreactor forever. Upon proof of concept, the team will want to scale up to bring effective treatments to more people. Accomplishing that scalability easily and cost effectively requires planning for it from the earliest stages (Figure 1).

Figure 1: Seamlessly integrated solutions support scalability.
A key element to unlocking scalability and speed to market is flexible operations. From the earliest stages, teams should design automation into their processes to reduce errors, allow for fast and seamless changes, and promote simpler operation and iteration across development. The worst-case scenario is developing a process designed around equipment and software that must be scrapped and rebuilt upon scaling because it cannot operate at that scale, whether scaling up or out. However, even if the team can create a scaled solution via complex engineering, such a configuration will create challenges of its own.
Planning for the future
Fortunately, modern technologies are helping cell and gene therapy teams take a born digital approach to futureproof their processes. Emerging methodologies are making it possible for teams to swap equipment in and out of their processes more easily as changes become necessary. By selecting solutions that support these flexible technologies from the earliest stages, teams are dramatically simplifying their adjustments and building more modular processes.
In addition, many teams are exploring software solutions like PKM software to help them manage their data end-to-end across the development pipeline. The right PKM solution helps ensure continuity, consistency, quality, and security of data at every stage, not only making it easier to move across the technology transfer process, but also providing support in late stages where auditing earlier steps in development may be necessary (Figure 2).
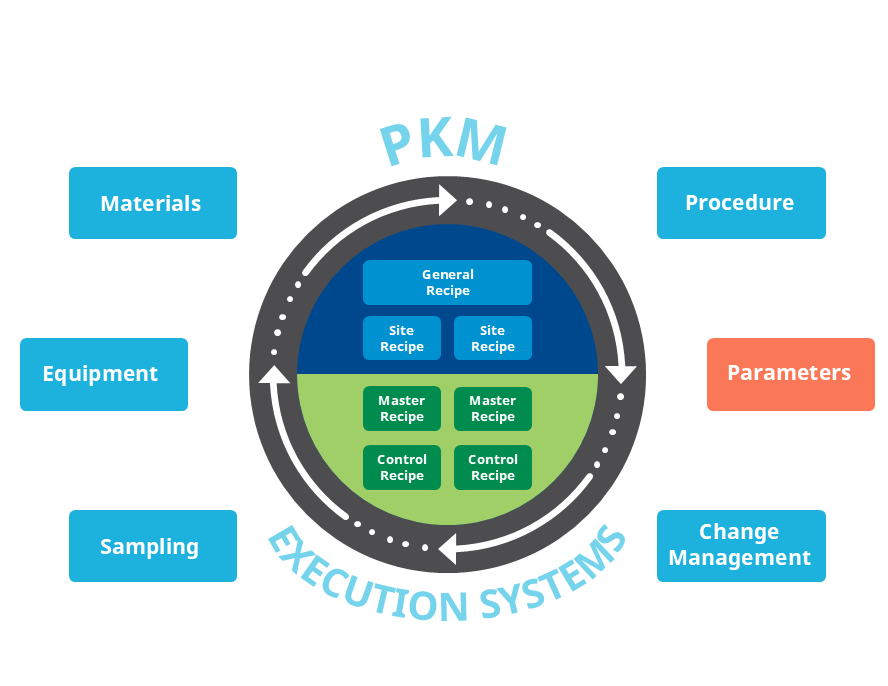
Figure 2: PKM software accelerates technology transfer, standardizes processes, and boosts flexibility.
Building for purpose
There are many new technologies that cell and gene therapy teams are employing as part of their automation strategy to simplify and streamline development and operation. However, the most effective teams are upleveling that process with a born-digital strategy designed to lock in intuitive operations and seamless integration from the earliest stages.
These organizations are requiring automation solutions providers to help them build future-proofed systems through delivery of natively integrated solutions that simplify operation and are built around the key technologies necessary to unlock modularity and flexibility. It is possible to find solutions that promote simplicity rather than complexity—if teams plan for those strategies from the earliest stages of development.
All figures courtesy of Emerson

Kristel Biehler
About the author
Kristel Biehler is vice president of life sciences for Emerson’s process systems and solutions business where she leads the day-to-day business activities in sales, operations, and technology that serve the life science industries. In her previous role at Emerson, she was the automation solutions vice president of sales for the western United States where she led teams that helped customers identify, architect, and implement automation and digital strategies across a wide range of industries. Kristel Biehler started her career with Emerson in 1998. She holds a bachelor’s degree in mechanical engineering from the University of Utah. Prior to Emerson, she worked for Sorex Medical as an automation engineer.
The post Seamless scaling: The role of smart automation in CGT manufacturing appeared first on Pharmaceutical Processing World.
Qosina, AdvantaPure collaborate on pharmaceutical manufacturing 31 Mar 2025, 6:57 pm
 Qosina announced today that it entered into a collaboration with AdvantaPure to enhance its selection of solutions for pharmaceutical manufacturing.
Qosina announced today that it entered into a collaboration with AdvantaPure to enhance its selection of solutions for pharmaceutical manufacturing.
AdvantaPure, the high-purity products division of NewAge Industries, anticipates and satisfies the evolving needs of the pharmaceutical and biopharmaceutical manufacturing industries. As an official distribution partner for AdvantaPure, Qosina now expands its tubing portfolio with more than 60 new tubing options. This further enhances its offerings for engineers, designers and manufacturers.
The company will showcase this partnership and the AdvantaPure tubing offering at its booth (3317) during the Interphex trade show in New York this week. Attendees can explore the breadth of AdvantaPure tubing available through Qosina.
“Our collaboration with AdvantaPure marks an important step forward for Qosina as we continue to expand our offerings and provide greater value to our customers,” said Lee Pochter, CEO. “This partnership underscores Qosina’s commitment to meeting the critical needs of the life sciences industry. With the addition of AdvantaPure’s trusted tubing solutions, Qosina is strengthening its ability to offer off-the-shelf convenience and flexibility to customers worldwide.”
The post Qosina, AdvantaPure collaborate on pharmaceutical manufacturing appeared first on Pharmaceutical Processing World.
How Cegeka tackles GxP validation in pharmaceutical ERP projects 28 Mar 2025, 6:39 pm
 Dominique De Vos, Global Solution Manager Pharma & Life Sciences at Cegeka has an equation to make sense of digital transformation in the pharma landscape and beyond. NO + OT = CNO. In other words, “New Organization plus Old Technology equals Costly New Organization.” The equation is a reminder that legacy systems are often incompatible with modern pharmaceutical operations.
Dominique De Vos, Global Solution Manager Pharma & Life Sciences at Cegeka has an equation to make sense of digital transformation in the pharma landscape and beyond. NO + OT = CNO. In other words, “New Organization plus Old Technology equals Costly New Organization.” The equation is a reminder that legacy systems are often incompatible with modern pharmaceutical operations.
Pharmaceutical manufacturers face a convergence of intense pressures. In one corner, intensifying global regulations (like evolving GxP standards and serialization mandates) demand constant adaptation. In another, increasingly complex multi-stage supply chains require precise temperature control and traceability. Compounding these challenges is the urgent need to modernize costly, often siloed, legacy systems.
Successfully navigating this landscape requires more than generic IT solutions; it demands digital systems deeply integrated into core operations. Enterprise Resource Planning (ERP) platforms like Microsoft Dynamics 365 are crucial for improving efficiency and ensuring compliance. Yet implementing and validating these systems within the highly regulated life sciences sector is where standard approaches often falter. It requires specific expertise not just in the software, but in GxP compliance (Good Manufacturing/Distribution/Clinical/Laboratory Practices), Computerized System Validation (CSV), quality management integration, and the workflows of pharmaceutical production and batch release. To understand how companies can bridge this gap, we spoke with Dominique De Vos, Global Solution Manager Pharma & Life Sciences at Cegeka, who will share thoughts on these subjects at PHARMAP 2025 (April 14-15, Berlin) on tackling common implementation hurdles, ensuring rigorous GxP validation, supporting international scalability, and using digital tools to directly address these operational and compliance hurdles in pharmaceutical environments.
Can you share an overview of how these ERP implementations have specifically addressed the unique challenges of pharmaceutical manufacturing and packaging? What are common challenges you’ve helped pharma clients overcome with such implementations?

Dominique De Vos
Dominique: Pharmaceutical manufacturing and packaging present a unique set of challenges, particularly as companies expand globally and navigate increasingly complex regulatory landscapes. At Cegeka, we understand that scaling operations while maintaining compliance is crucial for our clients. Implementing Microsoft Dynamics 365 with our specialized Cegeka Pharma & Life Sciences for Dynamics 365 solution allows us to address these challenges through centralized data management, automated compliance controls, and enhanced supply chain visibility.
A significant 43% of executives in pharma are prioritizing global expansion and new product launches over the next two years, which requires managing diverse regulatory environments across multiple regions. Microsoft Dynamics 365, in combination with Cegeka Pharma & Life Sciences for Dynamics 365 solution, enables companies to harmonize operations, ensuring adherence with global standards like FDA and EMA, while supporting smooth cross-border supply chain coordination.
With automated workflows, Microsoft Dynamics 365 ensures that companies can navigate evolving regulations with ease, reducing the risk of regulatory penalties, product recalls, or operational disruptions.
Supply chain visibility is another critical challenge, especially with time-sensitive products. Research shows that 87% of pharmaceutical companies lack complete visibility into product conditions during the last mile of delivery, leading to inefficiencies and delays. Microsoft Dynamics 365 enhances real-time tracking and supply chain insights, enabling companies to respond quickly to disruptions, maintain inventory accuracy, and ensure timely delivery of life-saving products, even across complex global networks.
Our Cegeka Pharma & Life Sciences solution combines deep industry expertise with a tailored ERP platform. It simplifies regulatory conformity, enhances supply chain visibility, and scales effortlessly to meet business growth. This comprehensive approach enables our clients to operate efficiently, respond to market demands, and maintain compliance across global operations.
In your recent LinkedIn posts, you’ve highlighted Cegeka’s work with companies. Could you share some examples of how digital transformation is improving efficiency or compliance in pharmaceutical production environments?
Dominique: Digital transformation is a game-changer for pharmaceutical production, allowing companies to enhance operational efficiency while staying ahead of evolving regulatory demands. We’ve seen firsthand how Microsoft Dynamics 365 delivers real-time insights, process automation, and robust frameworks that help our clients navigate these challenges.
A compelling example is our collaboration with Eurofins. Facing the limitations of an outdated ERP system, Eurofins needed a solution that could streamline global operations while maintaining regulatory adherence across multiple European sites. By implementing Microsoft Dynamics 365, we centralized Eurofins’ data management and improved their ability to respond swiftly to regulatory changes. This approach has simplified audits and has reduced the administrative burden on their teams, allowing them to focus on core business activities.
Another significant challenge is supply chain visibility and responsiveness, particularly for companies managing time-sensitive products. A recent project with a global radiopharmaceutical leader underscored the need for an integrated supply chain. With products that have a shelf life of just 48 hours, precise coordination across business units was essential to ensure timely delivery to patients worldwide. By implementing Microsoft Dynamics 365 with our Cegeka Pharma & Life Sciences solution, they gained real-time supply chain tracking and improved operational coordination, ensuring life-saving treatments reached patients on time across more than 60 countries.
In the case of our customer Nyxoah, digital transformation has enabled scalable, efficient processes across their expanding global footprint. By integrating their operations on Microsoft Dynamics 365, we established automated workflows and a single source of truth for regulatory reporting. This has allowed their teams to respond quickly to new market demands without compromising quality or compliance.
These examples highlight how our digital transformation initiatives empower pharmaceutical companies to adapt to industry demands, enhance efficiency, and maintain the highest compliance standards in an increasingly complex environment.
GxP compliance appears to be a key focus in your work. How is Cegeka helping pharmaceutical manufacturers ensure their digital systems meet these strict regulatory requirements while still enabling innovation?
Dominique: At Cegeka, we recognize that adhering to GxP guidelines—which cover a range of Good Practices, including manufacturing (GMP), clinical (GCP), laboratory (GLP), and distribution (GDP)—is essential for pharmaceutical manufacturers, particularly as they undergo digital transformation. While Microsoft Dynamics 365 provides a strong foundation for GxP compliance with built-in features like audit trails, data integrity controls, role-based access, and document management, it is essential to configure and validate the system to meet specific regulatory requirements.
This is where our partnership with Epista Life Science adds significant value. As specialists in Computerized System Validation (CSV) and GxP compliance, Epista ensures that every implementation meets stringent regulatory standards while supporting operational efficiency.
In close collaboration, we help pharmaceutical companies implement Microsoft Dynamics 365 in a way that ensures compliance while enabling innovation. Our combined approach includes validation support throughout the system lifecycle, from design through implementation and ongoing operation. By leveraging Epista’s deep expertise in CSV and GxP, we ensure that every aspect of the system is validated, and that all processes – such as batch production, traceability, and quality control – are fully compliant with regulatory standards.
Moreover, Microsoft Dynamics 365 offers the flexibility to drive innovation without compromising regulatory adherence. It allows manufacturers to scale efficiently, optimize supply chains, and improve production processes, all while maintaining the high regulatory standards required by the industry. The system’s adaptability ensures that manufacturers can implement new technologies and improve operational efficiencies without putting GxP compliance at risk.
In summary, Cegeka, in partnership with Epista Life Science, provides comprehensive support to pharmaceutical manufacturers to ensure GxP compliance with Microsoft Dynamics 365. Through CSV validation, automated controls, and our combined expertise, we enable clients to innovate confidently within a highly regulated environment, ensuring they remain compliant while achieving operational excellence.
How do you foresee AI and Copilot transforming pharmaceutical manufacturing operations in the next 2-3 years? And regarding cybersecurity, what is the current state of awareness within the sector?
Dominique: The potential of AI and Copilot technologies in pharmaceutical manufacturing is immense, and we’re only beginning to see their impact. In the next few years, these tools will help automate routine tasks, allowing professionals to focus on more meaningful work and reducing the risk of burnout—a common challenge in healthcare.
AI will significantly improve data integrity, security, and validity, which is crucial in a world that is increasingly focused on patient-centric care. Technologies like Copilot integrated into Microsoft Dynamics 365 will ensure better regulatory adherence by offering real-time assistance, reducing errors, and improving decision-making on the production floor. The Healthcare Information and Management Systems Society (HIMSS) www.himss.org is supporting this AI revolution by offering global guidance through their Responsible AI Policy Principles and providing professional development courses on AI, helping healthcare and pharmaceutical companies navigate the complexities of an implementation.
When it comes to cybersecurity, awareness in the pharmaceutical industry has significantly improved, especially as the sector faces increasing cyber threats. HIMSS is actively engaged in promoting cybersecurity best practices, including organizing forums with experts and releasing the annual Healthcare Cybersecurity Survey, which provides valuable insights into current trends, challenges, and strategies.
Software validation is often a significant hurdle for pharmaceutical companies. Could you explain how Cegeka approaches this process to reduce the burden on manufacturers while maintaining compliance?
Dominique: The answer to this question connects to question 3 about the end-to-end attention for regulatory requirements. In summary, Cegeka, in partnership with Epista Life Science, provides comprehensive support to pharmaceutical manufacturers to ensure GxP compliance with Microsoft Dynamics 365. Through CSV validation, automated controls, and our combined expertise, we enable clients to innovate confidently within a highly regulated environment, ensuring they remain compliant while achieving operational excellence.
Epista Life Science did not only verify our Cegeka Pharma & Life Sciences solution, but also the way we implement our solution at Pharma & Life Sciences manufacturing companies. Our implementation methodology has been validated in such a way that our project deliverables are fully aligned with validation deliverables according to GAMP5 recommendations, which allows our customer to pass (often unexpected or unannounced) audits by local and/or international authorities.
This way, we unburden our customers by guaranteeing that we keep our solution in a continuously compliant state, taking care of validation and testing throughout the entire lifespan of the solution, and in particular when platform releases are being launched by Microsoft twice per year.
Many pharmaceutical companies are expanding internationally. What are the key ERP considerations when supporting global operations across different regulatory environments?
Dominique: 43% of pharma executives prioritize expanding internationally and launching new products in the next 2 years, which clearly shows this is a top priority for many pharma manufacturing companies. Our global customers need to both scale their operations across diverse regulatory environments, and to manage R&D pipelines and to accelerate time-to-market, which requires seamless cross-border supply chain coordination.
The Microsoft Dynamics 365 Finance & Supply Chain Management solution provides out-of-the-box localizations for most countries worldwide, covering the country-specific accounting and legislation requirements. Moreover, this solution is designed to provide global capabilities, making it suitable for multinational organizations operating in diverse regions. Microsoft Dynamics 365 Finance & Supply Chain Management supports multiple languages, allowing users to interact with the system in their preferred language.
What impact is the drive toward sustainability having on pharmaceutical manufacturing technology, and how are you/Cegeka addressing these evolving requirements?
Dominique: Pharmaceutical companies have a considerable environmental impact in terms of waste disposal, CO2 emissions, plastic usage, and air pollution, water and energy consumption (including those related to transportation and refrigeration of drugs). In fact, pharmaceutical industry CO₂ emissions are 13% higher than those of the automotive sector, according to data from 2019. As sustainability becomes a global priority, the industry is under increasing pressure to reduce its environmental footprint while maintaining operational efficiency and regulatory conformity.
At Cegeka, we are helping our pharmaceutical clients embrace sustainability through digital transformation. By implementing Microsoft Dynamics 365, we are enabling manufacturers to optimize their operations, enhance resource management, and track energy usage more effectively. For example, AI-powered analytics within these systems can help identify inefficiencies in production processes and suggest more sustainable alternatives.
One key focus is supporting sustainable supply chain management. Through advanced tracking and reporting tools, we help pharmaceutical manufacturers monitor the carbon impact of their entire supply chain—from raw material sourcing to final product delivery. This allows companies to optimize transportation routes, reduce waste, and ensure compliance with global sustainability standards like ISO 14001 and emerging ESG regulations.
Additionally, our partnership with Epista Life Science enhances these efforts by ensuring that all digital systems comply with GxP regulations while supporting environmentally responsible practices. This balance between compliance and sustainability is critical as companies work toward greener operations without compromising data integrity or product safety.
Beyond technology, we recognize that true sustainability requires a cultural shift within organizations. We assist our clients in establishing standardized processes, reward programs, and staff training to encourage sustainable behaviors at every level. This holistic approach helps pharmaceutical companies not only reduce their environmental impact but also align with evolving global expectations around corporate responsibility.
For pharmaceutical manufacturers still using legacy systems, what would you identify as the most compelling reasons to undertake digital transformation now rather than later?
Dominique: I would like to summarize my point of view with the following ‘riddle’ :
NO + OT = CNO, which stands for NEW ORGANIZATION + OLD TECHNOLOGY = COSTLY NEW ORGANIZATION.
Delaying digital transformation in pharmaceutical manufacturing is becoming an increasingly risky and costly decision. Legacy systems, while familiar, often lack the scalability, compliance capabilities, and efficiency needed to navigate today’s fast-changing regulatory and operational landscape. There are several urgent reasons why transforming now is not just beneficial—but essential.
First, regulatory pressures are intensifying globally. With 53% of pharma leaders identifying increasing regulation as the #1 external risk (Gartner, 2024), outdated systems that rely on manual processes struggle to keep pace with evolving standards like FDA 21 CFR Part 11, EMA Annex 11, and new ESG reporting requirements. Modern ERP systems—such as Microsoft Dynamics 365—offer automated compliance controls, audit trails, and real-time reporting, reducing the risk of regulatory violations and costly regulatory penalties.
Second, operational agility is crucial in a competitive market. Pharmaceutical companies are under pressure to accelerate time-to-market while managing supply chain complexities and demand fluctuations. Legacy systems often operate in silos, limiting real-time visibility and creating bottlenecks in production planning and inventory management. Digital platforms provide end-to-end transparency, enabling manufacturers to respond more quickly to market changes, supply disruptions, or new product launches—a critical advantage as 43% of pharma executives prioritize global expansion over the next two years (Gartner, 2024).
Another compelling reason is data integrity and security. With the rise of cyber threats targeting the pharmaceutical sector, legacy systems—often lacking advanced security protocols—pose a significant vulnerability. Modern cloud-based ERP solutions provide robust cybersecurity measures, data encryption, and access controls to protect sensitive patient data and intellectual property while ensuring business continuity.
Finally, the cost of inaction is rising. Maintaining and customizing outdated systems is not only expensive but also inflexible when it comes to integrating emerging technologies like AI and machine learning. With tools like Microsoft Copilot, pharmaceutical manufacturers can automate manual tasks, enhance decision-making, and improve process efficiency—driving both cost savings and innovation.
At Cegeka, we work closely with pharmaceutical companies to make digital transformation a strategic enabler rather than just a technical upgrade. By adopting modern systems now, companies can future-proof their operations, ensure regulatory adherence, and gain the agility needed to thrive in an increasingly digital and regulated environment. Waiting only increases the risks and costs—transforming now is the smarter, safer choice.
Hear more from Dominique De Vos on tapping ERP for pharmaceutical manufacturing challenges, GxP compliance, and digital innovation at PHARMAP 2025, taking place April 14-15 in Berlin.
The post How Cegeka tackles GxP validation in pharmaceutical ERP projects appeared first on Pharmaceutical Processing World.
Merck KGaA Q&A: Solving pharma’s ‘make’ step with SYNTHIA AI synthesis planning 28 Mar 2025, 6:08 pm
 The buzz around AI in drug discovery often centers on generating new molecular ideas. But as computational chemists know, an in silico design is only valuable if it can be efficiently synthesized in the lab and is manufacturable after that. Merck KGaA, Darmstadt, Germany, with its roots in both chemistry (via Sigma-Aldrich) and life science technology, is tackling this “make” step. Its SYNTHIA Retrosynthesis Software uses AI to map out viable synthetic pathways, directly connecting computational design to the practicalities of the bench and potential scale-up, even linking to commercially available starting materials. Ewa Gajewska, Head of Product Management for SYNTHIA, who is speaking at PHARMAP 2025 (April 14-15, Berlin), explains how the software streamlines this critical transition.
The buzz around AI in drug discovery often centers on generating new molecular ideas. But as computational chemists know, an in silico design is only valuable if it can be efficiently synthesized in the lab and is manufacturable after that. Merck KGaA, Darmstadt, Germany, with its roots in both chemistry (via Sigma-Aldrich) and life science technology, is tackling this “make” step. Its SYNTHIA Retrosynthesis Software uses AI to map out viable synthetic pathways, directly connecting computational design to the practicalities of the bench and potential scale-up, even linking to commercially available starting materials. Ewa Gajewska, Head of Product Management for SYNTHIA, who is speaking at PHARMAP 2025 (April 14-15, Berlin), explains how the software streamlines this critical transition.
In the following interview, Gajewska touchs on how AI-driven retrosynthesis is transforming drug development from lab to production. Responses have been lightly edited for brevity.
As Head of Product Management for SYNTHIA at Merck KGaA, Darmstadt, Germany, could you explain how SYNTHIA Retrosynthesis Software is changing the way pharmaceutical companies approach drug development and manufacturing?
Ewa Gajewska
Gajewska: SYNTHIA Retrosynthesis Software changes drug development by automating retrosynthetic analysis. Traditionally, chemists spent a lot of time manually mapping out potential synthetic routes. With advanced algorithms and AI, SYNTHIA handles this process quickly, generating and comparing many possible pathways, which saves time and money.
SYNTHIA also uncovers creative synthetic options. It uses a large database of expert-coded reaction rules and computational models to spot routes that might not be obvious to human chemists—especially for novel compounds. This approach can lead to faster, more scalable manufacturing, and SYNTHIA’s forward synthesis feature can even predict entirely new drug structures.
SYNTHIA aligns with the growing push for sustainable pharmaceutical manufacturing by flagging routes that use fewer steps or less hazardous reagents. This helps meet regulatory standards and supports environmental goals. At Merck KGaA, Darmstadt, Germany, we find that SYNTHIA can accelerate both innovation and efficiency.
By blending human expertise and AI, SYNTHIA helps companies innovate faster, cut costs, and adopt greener practices. From early research to industrial-scale production, it’s a powerful tool for discovering new therapies.
The 2024 webinar “AI meets chemistry” showcased how SYNTHIA integrates with AI systems for drug discovery. Can you share your perspective on how this technology helps bridge the gap between drug discovery and commercial-scale manufacturing?
Gajewska: As highlighted in the webinar, SYNTHIA automates the search for ideal synthetic pathways, speeding up planning and reducing human error. It ensures these routes are practical and scalable for industrial use.
A key feature is SYNTHIA’s Synthetic Accessibility Score (SAS) API, which rates a molecule’s complexity so researchers can focus on targets that are easier to make. This helps prioritize promising candidates, saving time and resources. This focus on feasibility helps SYNTHIA meet the industry’s demand for efficiency across discovery and manufacturing.
What is the current maturity level you see with AI in chemistry in pharma contexts in 2025?
Gajewska: AI in chemistry is advanced but still growing. Platforms like SYNTHIA significantly improve retrosynthesis and molecule selection, yet full integration into every step of drug development is ongoing. Reaction optimization still needs more reliability, and labs must adapt to incorporate AI tools smoothly.
AI in chemistry isn’t just a novelty—it’s becoming a standard part of R&D. Companies use AI-driven systems to accelerate discovery, refine synthetic routes, and control costs. By 2025, we expect AI to drive breakthroughs at a scale once thought impossible.
What specific manufacturing or production challenges can SYNTHIA help solve that traditional approaches cannot address as effectively?
Gajewska: SYNTHIA tackles several issues with scaling up complex molecules. Manual retrosynthesis can be slow and prone to mistakes, but SYNTHIA automates it, cutting planning time and offering multiple viable routes. It also evaluates cost and scalability from the start, minimizing headaches later on. The Synthetic Accessibility Score (SAS) API guides teams to molecules that are simpler to produce, reducing wasted time. For time-sensitive projects such as new antiviral drugs, SYNTHIA speeds up the path from discovery to production. Plus, it considers reagent availability and cost, helping teams plan around real-world manufacturing constraints.
How are pharmaceutical companies using SYNTHIA to address sustainability concerns in their manufacturing processes? Can AI-driven synthesis planning help reduce waste and resource consumption?
Gajewska: Many companies, including GSK, use SYNTHIA to design more sustainable synthetic pathways that produce less waste and require fewer or safer reagents. Because SYNTHIA maps out greener routes, it cuts trial-and-error efforts and helps researchers choose sustainable materials. This saves energy, lowers environmental impact, and keeps production efficient.
our software helps plan chemical syntheses, but implementation often requires adaptation for manufacturing scale. Can you say more on how SYNTHIA helps scientists and engineers make this transition from lab to production scale?
Gajewska: SYNTHIA goes beyond proof-of-concept. It checks for cost-efficiency, reproducibility, and the availability of reagents in bulk. By comparing multiple routes, SYNTHIA highlights the ones best suited for large-scale production. It factors in reaction conditions and streamlines the process to reduce bottlenecks as you scale up. SYNTHIA also promotes collaboration by letting teams share synthetic plans, connecting research and manufacturing more smoothly.
In your recent publications, you’ve explored computer-generated “synthetic contingency” plans for supply chain challenges. How can pharmaceutical manufacturers tap this capability to enhance resilience in their production processes?
Gajewska: SYNTHIA can build “synthetic contingency” plans that prepare companies for supply chain surprises. It spots alternative routes relying on more common, cheaper reagents, so you’re less vulnerable if a key material is in short supply. This flexibility keeps production stable and helps you adapt quickly to sudden changes in availability or costs.
What role do you see AI and computational chemistry tools like SYNTHIA playing in the future of pharmaceutical packaging innovation?
Gajewska: Eventually, we’ll see AI-enabled packaging, like embedded sensors that track drug stability or indicate expiration. By combining computational chemistry with materials science, drugmakers can create packaging that’s both sustainable and responsive to real-world conditions.
How is SYNTHIA evolving to meet the specific needs of the pharmaceutical manufacturing sector, especially regarding regulatory compliance and quality control?
Gajewska: SYNTHIA pinpoints synthetic routes that favor safety, reproducibility, and compliance with regulations. It can flag potential side reactions or impurities, helping refine processes to ensure product quality. Its emphasis on scalability and cost-effectiveness supports stable and efficient production. As digital integration progresses, SYNTHIA will continue to smooth out workflows, making it a key tool in pharmaceutical manufacturing.
Hear more from Ewa Gajewska on the role of AI and tools like SYNTHIA in pharmaceutical development and manufacturing at PHARMAP 2025 (April 14-15, Berlin).
The post Merck KGaA Q&A: Solving pharma’s ‘make’ step with SYNTHIA AI synthesis planning appeared first on Pharmaceutical Processing World.
J&J breaks ground on $2B manufacturing facility in North Carolina 21 Mar 2025, 8:12 pm
 Johnson & Johnson today announced it broke ground on a $2 billion biologics manufacturing facility in Wilson, N.C., a small portion of the company’s $55 billion U.S. investment initiative over the next four years.
Johnson & Johnson today announced it broke ground on a $2 billion biologics manufacturing facility in Wilson, N.C., a small portion of the company’s $55 billion U.S. investment initiative over the next four years.
The 500,000 ft² facility will expand the company’s capacity to develop and produce advanced therapies for oncology, immunology and neurological diseases. Once operational, it is expected to employ more than 500 workers and contribute $3 billion in economic impact across North Carolina during its first decade.
“We are pleased to make this significant investment in our manufacturing network in the United States. This state-of-the-art biologics facility in North Carolina will help Johnson & Johnson to accelerate the delivery of our portfolio and pipeline of transformational medicines for patients,” said Jennifer Taubert, executive VP and worldwide chair of J&J Innovative Medicine. “This $2 billion investment will bring more than 5,000 high-wage manufacturing and construction jobs to North Carolina and the partnerships we are forging in the community will support local educational initiatives to develop the workforce of the future.”
The Wilson facility is part of Johnson & Johnson’s broader plan to expand its manufacturing and R&D capabilities in the U.S., where it already operates 22 manufacturing sites. The company estimates that approximately 5,000 construction jobs will be created during the development of the North Carolina site alone.
North Carolina Gov. Josh Stein welcomed the company’s presence in the state.
“Wilson is an important life sciences hub, and I am excited to welcome Johnson & Johnson as it expands its manufacturing footprint in our state,” said Stein. “I look forward to seeing the impact this investment will have on medical innovation and North Carolina’s workforce.”
Collaborating with the community
In addition to new jobs and infrastructure, the company announced workforce development initiatives targeting local students. These include expanded STEM programs in Wilson County Schools through a partnership with the Smithsonian Science Education Center and the rollout of the BioWork certificate program in all Wilson County high schools. The program, developed with the North Carolina Biotechnology Center and Wilson Community College, will equip students with the skills needed for careers in the life sciences industry.
“Johnson & Johnson is a recognized industry leader in pharmaceutical research, development and manufacturing, and we are proud to be part of the Wilson community,” said Dapo Ajayi, VP of Innovative Medicine Supply Chain at Johnson & Johnson. “This is a major investment and an exciting time as we start construction on this advanced manufacturing facility, using the latest technology and building a highly skilled workforce to deliver on our promise to patients.”
The groundbreaking follows Johnson & Johnson’s announcement of its expanded U.S. investment strategy, which includes advanced manufacturing, digital technologies, and research infrastructure focused on oncology, immunology, neuroscience, cardiovascular disease, and robotic surgery.
The post J&J breaks ground on $2B manufacturing facility in North Carolina appeared first on Pharmaceutical Processing World.
How real-time sensors and data-savvy can cut energy waste in pharma 20 Mar 2025, 5:25 pm

[Image courtesy of Adobe Stock]
Such sensors and technologies offer more than mere compliance checkpoints—they provide data points in the pharmaceutical industry’s growing sustainability strategy while offering growing opportunities for automation. “Pharma is a perfect fit for our automated approach,” explains Stanislav Kazanov, Head of Sustainability at Innowise, who will speak at PHARMAP 2025 in Berlin. “We can easily monitor things like energy use in bioreactors, water consumption in purification processes, and waste generation from API production. This data-driven approach streamlines compliance documentation and helps identify resource-intensive processes like lyophilization for improvement.”
Kazanov goes on to say that “pharma’s GMP requirements are a real asset.” He continued: “The thorough validation process ensures the environmental data we collect is highly reliable.”
Breaking down big-picture challenges
 Kazanov’s employer Innowise is an international software development compan expanding its footprint in the pharmaceutical sector by connecting these regulatory requirements with environmental goals. Kazanov brings a unique perspective to sustainability, having spent years as a data engineer before taking on his current role. “Coming from data engineering, I know the impact of clear, measurable information,” he notes. “In sustainability, it’s easy to get lost in big-picture ideas. We break environmental challenges into manageable pieces with software that tracks energy use and waste, so strategies are based on hard numbers, not just good intentions.”
Kazanov’s employer Innowise is an international software development compan expanding its footprint in the pharmaceutical sector by connecting these regulatory requirements with environmental goals. Kazanov brings a unique perspective to sustainability, having spent years as a data engineer before taking on his current role. “Coming from data engineering, I know the impact of clear, measurable information,” he notes. “In sustainability, it’s easy to get lost in big-picture ideas. We break environmental challenges into manageable pieces with software that tracks energy use and waste, so strategies are based on hard numbers, not just good intentions.”
Bridging compliance, data analytics, and sustainability often brings together teams who rarely collaborated in the past—like production line engineers, environmental health and safety officers, and IT specialists. Kazanov notes that when these departments share a unified data dashboard, it not only streamlines decision-making but also fosters a culture of continuous improvement.
This data-focused approach helps pharmaceutical companies tackle what Kazanov identifies as their three biggest sustainability challenges: “optimization of energy use in manufacturing and cold storage, finding new ways to manage waste—particularly chemical byproducts and packaging—and optimization of the supply chain to reduce transportation emissions.”

Stanislav Kazanov
The key, according to Kazanov, is developing solutions that make a real impact while working within the industry’s strict regulatory framework. “Pharmaceutical manufacturing definitely has a unique set of sustainability challenges. The key is finding solutions that work—read: make impact—within the existing regulatory framework.”
Investments in a green future
The pharmaceutical industry’s increasing focus on sustainability comes at an opportune time. “The news that 92% of CFOs plan to increase sustainability spending in 2025 is incredibly encouraging, and it opens the door for strategic investments in pharma,” Kazanov says.
He recommends manufacturers focus their investments in three key areas: energy-efficient technologies like advanced cooling systems and continuous processing equipment; digital tools that streamline sustainability tracking and reporting; and innovations in green chemistry, including more efficient processes and sustainable packaging.
“For pharma manufacturers, some of the smartest investments will be in energy-efficient technologies that can cut operating costs and reduce environmental impact simultaneously,” Kazanov explains. “Digital tools that speed up sustainability tracking and reporting are another good bet—they save time and build credibility with stakeholders.”
Converting data into strategic action
At the heart of Innowise’s approach are sustainability applications and Environmental Product Declaration (EPD) tools designed specifically for pharmaceutical companies. Rather than simply collecting data, these technologies provide actionable insights.
“The sustainability apps and EPD tools we’ve developed at Innowise are mostly about providing insights,” says Kazanov. “For pharmaceutical companies, this means understanding the environmental impact of their products at every stage. The app gives a real-time view of emissions, water use, and waste, highlighting areas for targeted improvement. The EPD tools then help communicate that progress to regulators and customers.”
This real-time capability represents a significant advancement for an industry accustomed to retrospective reporting. “Today, real-time data analytics provides a critical advantage for pharma companies,” Kazanov points out. “They get a constant stream of information on everything from emissions and resource use to critical quality parameters like temperature and contamination. This allows pharma manufacturers to address issues immediately, adapt to changing ESG expectations, and maintain GMP compliance all at the same time.”
Despite the technological advances in the industry, Kazanov sees one area with tremendous untapped potential: artificial intelligence. “It’s surprising that only 6% of companies use AI for sustainability. There’s so much potential, especially in pharma,” he says.
For instance, predictive AI models can anticipate maintenance needs for pumps and agitators, preventing downtime and reducing unnecessary power consumption. Over time, such targeted interventions can accumulate into notable environmental and cost benefits.
He outlines both short-term and long-term applications for AI in pharmaceutical sustainability. “For a quick start, AI can analyze equipment performance to identify energy savings or automate ESG reporting. But the real potential lies in longer-term initiatives: optimization of drug production, supply chain needs forecasts, and even simulation of environmental impact before manufacturing begins.”
The future of green pharmaceutical manufacturing
Looking ahead, Kazanov envisions significant transformation in the pharmaceutical industry’s approach to sustainability over the next few years. “What will pharmaceutical sustainability look like in 3-5 years? I predict a shift towards continuous manufacturing, a rise in biodegradable and smart packaging, AI-powered optimization, and perhaps blockchain integration in supply chains,” he forecasts.
These technologies, he believes, have the potential to fundamentally change how the industry approaches environmental responsibility—creating a future where regulatory compliance and sustainability goals aren’t competing priorities but complementary objectives achieved through the same data-driven processes.
“I truly believe these technologies have the potential to positively change the industry’s approach to environmental responsibility,” Kazanov concludes. “It’s about finding that balance between doing good for the planet and running a smart business.”
Stanislav Kazanov will be speaking at PHARMAP 2025 by BGS Pharmaceutical Events in Berlin on April 14-15, discussing Digital Intelligence in Green Drug Manufacturing.
The post How real-time sensors and data-savvy can cut energy waste in pharma appeared first on Pharmaceutical Processing World.
FDA approves additional manufacturing sites for Currax Pharma 20 Mar 2025, 3:53 pm
 Currax Pharmaceuticals today announced the FDA approved a second manufacturing site for its Contrave medication.
Currax Pharmaceuticals today announced the FDA approved a second manufacturing site for its Contrave medication.
The approval of the second site enhances the production capacity for the company’s oral weight loss medication, also marketed as Mysimba in Europe. Currax said it is investing in manufacturing and advancing clinical research.
“At Currax, we are steadfast in our commitment to ensuring that patients and healthcare providers have uninterrupted access to Contrave,” said Aaron Chesnut, VP of technical operations. “Securing a second FDA-approved drug product manufacturing site enhances our ability to respond to shifting industry conditions and safeguards against external factors that could impact production and distribution.”
Currax said the approval comes as demand for effective obesity treatments surges worldwide.
“As the obesity epidemic continues to rise, so does the need for dependable access to effective and affordable treatment options,” President and CEO George Hampton said. “As the only medication in the Reward System Modulator (RSR) class, Contrave is an important treatment option for physicians, particularly as the development programs continue to focus on the (GLP-1s).”
The post FDA approves additional manufacturing sites for Currax Pharma appeared first on Pharmaceutical Processing World.